2,6-二甲基-4-硝基吡啶 1-氧化物 | 4808-64-4
分子结构分类
中文名称
2,6-二甲基-4-硝基吡啶 1-氧化物
中文别名
2,6-二甲基-4-硝基吡啶氮氧化物;2,6-二甲基-4-硝基吡啶1-氧化物
英文名称
4-nitro-2,6-lutidine-1-oxide
英文别名
2,6-dimethyl-4-nitropyridine N-oxide;2,6-dimethyl-4-nitropyridine 1-oxide;4-nitro-2,6-lutidine N-oxide;2,6-dimethyl-4-nitro-1-oxidopyridin-1-ium
CAS
4808-64-4
化学式
C7H8N2O3
mdl
MFCD00023406
分子量
168.152
InChiKey
LFPUGENPUAERSG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:163 °C(Solv: ethanol (64-17-5))
-
沸点:413.8±40.0 °C(Predicted)
-
密度:1.30±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:12
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.285
-
拓扑面积:71.3
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2933399090
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2,6-Dimethyl-4-nitropyridine N-oxide
Synonyms: 2,6-Dimethyl-4-nitropyridin-1-ium-1-olate
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2,6-Dimethyl-4-nitropyridine N-oxide
CAS number: 4808-64-4
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
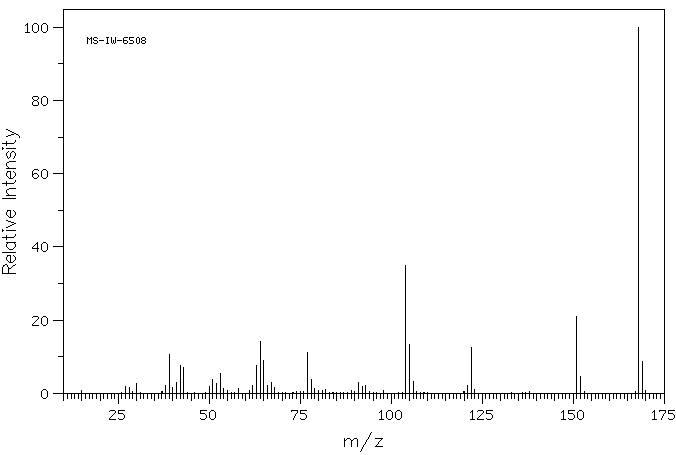
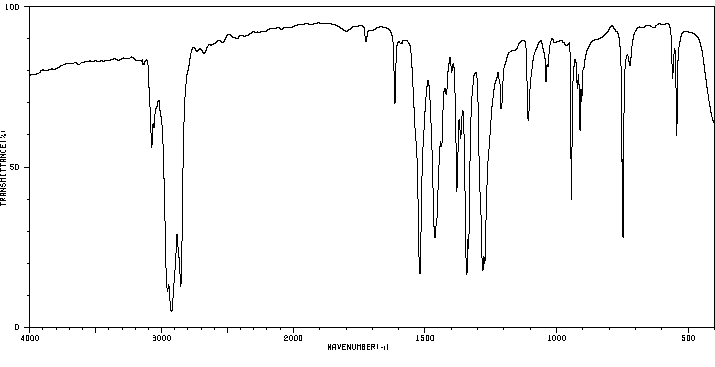
Molecular formula: C7H8N2O3
Molecular weight: 168.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2,6-Dimethyl-4-nitropyridine N-oxide
Synonyms: 2,6-Dimethyl-4-nitropyridin-1-ium-1-olate
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2,6-Dimethyl-4-nitropyridine N-oxide
CAS number: 4808-64-4
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H8N2O3
Molecular weight: 168.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-硝基吡啶-N-氧化物 4-Nitropyridine N-oxide 1124-33-0 C5H4N2O3 140.098 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,6-二甲基-4-硝基吡啶 4-nitro-2,6-lutidine 4913-57-9 C7H8N2O2 152.153 —— 1-hydroxy-2,6-dimethylpyridin-4-imine 3512-82-1 C7H10N2O 138.169 —— 6-methyl-4-nitro-pyridine-2-carbaldehyde 98549-98-5 C7H6N2O3 166.136 2,6-二(溴甲基)-4-硝基吡啶 2,6-bis(bromomethyl)-4-nitropyridine 358621-46-2 C7H6Br2N2O2 309.945
反应信息
-
作为反应物:描述:参考文献:名称:Novel nevirapine-like inhibitors with improved activity against NNRTI-resistant HIV: 8-heteroarylthiomethyldipyridodiazepinone derivatives摘要:A series of 8-heteroarylthiomethyidipyridodiazepinone derivatives were prepared and evaluated for their antiviral profile against wild type virus and the important K103N/Y181C mutant as an indicator for broad activity. 2,6-Dimethylpyridine derivative 16 was found to have a good pharmacokinetic profile in spite of poor metabolic stability in rat liver microsomes. (C) 2003 Published by Elsevier Ltd.DOI:10.1016/j.bmcl.2003.11.049
-
作为产物:参考文献:名称:稳定且刚性的类DTPA顺磁性标签,适用于体外和原位蛋白NMR分析。摘要:对顺磁性镧系元素离子具有高结合亲和力的配体的有机合成是对蛋白质产生顺磁性效应的有效方法。高分辨率NMR光谱中显示的这些顺磁效应是蛋白质和蛋白质-配体复合物的有价值的动态和结构约束。顺磁性标签通常包含金属螯合部分和用于蛋白质修饰的反应性基团。在这里,我们报告了两个新的类似DTPA的标签,即4PS-PyDTTA和4PS-6M-PyDTTA,它们可以位点特异性地连接到具有稳定硫醚键的蛋白质上。两种蛋白质标签加合物均形成稳定的镧系元素络合物,其结合亲和力和顺磁性张量相对于吡啶中的6-甲基是可调的。与伪接触位移(PCS)相比,评估了Gd(III)配合物对蛋白质标签加合物的顺磁弛豫增强(PRE)效果,结果表明4PS-PyDTTA和4PS-6M-PyDTTA标签都是刚性的,并且存在较高的质量的PRE,对于阐明蛋白质和蛋白质-配体复合物的动力学和相互作用至关重要。我们还显示这两个标签适用于原位蛋白NMR分析。DOI:10.1007/s10858-017-0160-3
文献信息
-
Chiral amine–imine ligands based on trans-2,5-disubstituted pyrrolidines and their application in the palladium-catalyzed allylic alkylation作者:Hongfeng Chen、James A. Sweet、Kin-Chung Lam、Arnold L. Rheingold、Dominic V. McGrathDOI:10.1016/j.tetasy.2009.07.010日期:2009.7moiety affords dramatic changes on the outcome of the stereochemistry. Evidence from various studies suggested that during the palladium-catalyzed allylic alkylation reaction, nucleophilic attack onto the 1,3-diphenylallyl moiety in the transition state occurs mainly trans to the pyridine ring of the less stable conformation of the palladium complexes.
-
一种用于蛋白质标记的二乙烯三胺四乙酸类顺磁探针申请人:南开大学公开号:CN106243020A公开(公告)日:2016-12-21
-
[EN] ISOTHIAZOLOQUINOLONES AND RELATED COMPOUNDS AS ANTI-INFECTIVE AGENTS<br/>[FR] ANTI-INFECTIEUX A BASE D'ISOTHIAZOLOQUINOLONES ET DE SELS CORRESPONDANTS申请人:ACHILLION PHARMACEUTICALS INC公开号:WO2005019228A1公开(公告)日:2005-03-03The invention provides compounds and salts of Formula (I) and Formula (II): which possess antimicrobial activity. The invention also provides novel synthetic intermediates useful in making compounds of Formula (I) and Formula (II). The variables A1, R2, R3, R5, R6, R7, A8 and R9 are defined herein. Certain compounds of Formula (I) and Formula (II) disclosed herein are potent and selective inhibitors of bacterial DNA synthesis and bacterial replication. The invention also provides antimicrobial compositions, including pharmaceutical compositions, containing one or more compounds of Formula (I) or Formula (II) and one or more carriers, excipients, or diluents. Such compositions may contain a compound of Formula (I) or Formula (II) as the only active agent or may contain a combination of a compound of Formula (I) or Formula (II) and one or more other active agents. The invention also provides methods for treating microbial infections in animals.本发明提供了具有抗菌活性的公式(I)和公式(II)的化合物及盐类:本发明还提供了用于制造公式(I)和公式(II)化合物的新的合成中间体。变量A1、R2、R3、R5、R6、R7、A8和R9在此文中定义。本文披露的某些公式(I)和公式(II)化合物是细菌DNA合成和细菌复制的强效和选择性抑制剂。本发明还提供了含有一种或多种公式(I)或公式(II)化合物以及一种或多种载体、辅料或稀释剂的抗菌组合物,包括药物组合物。这样的组合物可以只含有公式(I)或公式(II)的化合物作为唯一的活性成分,也可以含有公式(I)或公式(II)的化合物与一种或多种其他活性成分的组合。本发明还提供了用于治疗动物微生物感染的方法。
-
一种氯羟吡啶毒性杂质DCAL的制备及纯化方法
-
[EN] (3-HYDROXY-4-AMINO-BUTAN-2-YL) -3- (2-THIAZOL-2-YL-PYRROLIDINE-1-CARBONYL) BENZAMIDE DERIVATIVES AND RELATED COMPOUNDS AS BETA-SECRETASE INHIBITORS FOR TREATING<br/>[FR] DÉRIVÉS DE (3-HYDROXY-4-AMINO-BUTAN-2-YL) -3- (2-THIAZOL-2-YL-PYRROLIDINE-1-CARBONYL) BENZAMIDE ET COMPOSÉS ASSOCIÉS UTILISÉS EN TANT QU'INHIBITEURS DE LA BÊTA-SÉCRÉTASE POUR LE TRAITEMENT申请人:COMENTIS INC公开号:WO2009042694A1公开(公告)日:2009-04-02The present invention provides novel beta-secretase inhibitors and methods for their use, including methods of treating of Alzheimer's disease. (Formula)本发明提供了新颖的β-分泌酶抑制剂及其使用方法,包括用于治疗阿尔茨海默病的方法。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-硝基环己基乙酸酯
顺式-2-硝基-6-甲基环己酮
雷尼替丁杂质18
铝硝基甲烷三氯化物
钾离子载体III
重氮(硝基)甲烷
醛基-七聚乙二醇-叠氮
过氧亚甲基
辛腈,4-氟-4-硝基-7-羰基-
辛烷,1,2-二氯-1-硝基-
赤霉素A4+7(GA4:GA7=65:35)
苄哒唑
羟胺-四聚乙二醇-叠氮
羟胺-三乙二醇-叠氮
米索硝唑
磷酸十二醇酯
碘硝基甲烷
碘化e1,1-二甲基-4-羰基-3,5-二(3-苯基-2-亚丙烯基)哌啶正离子
硝酰胺
硝基脲银(I)复合物
硝基甲醇
硝基甲烷-d3
硝基甲烷-13C,d3
硝基甲烷-13C
硝基甲烷-(15)N
硝基甲烷
硝基甲基甲醇胺
硝基环辛烷
硝基环戊烷
硝基环戊基阴离子
硝基环庚烷
硝基环己烷锂盐
硝基环己烷钾盐
硝基环己烷
硝基环丁烷
硝基氨基甲酸
硝基新戊烷
硝基二乙醇胺
硝基乙醛缩二甲醇
硝基乙醛缩二乙醇
硝基乙腈
硝基乙烷-D5
硝基乙烷-1,1-d2
硝基乙烷
硝基乙烯
硝基丙烷
硝基丙二醛(E,E)-二肟
硝基丙二腈
硝基-(3-硝基-[4]吡啶基)-胺
硝乙醛肟