2-bromo-4,6-dichloro-5-methylbenzene-1,3-diol | 261958-57-0
中文名称
——
中文别名
——
英文名称
2-bromo-4,6-dichloro-5-methylbenzene-1,3-diol
英文别名
2-bromo-4,6-dichloro-5-methylresorcinol
CAS
261958-57-0
化学式
C7H5BrCl2O2
mdl
——
分子量
271.925
InChiKey
JMAYQAPCRHHHBV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:107 °C
-
沸点:280.3±35.0 °C(Predicted)
-
密度:1.906±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:12
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3,5-bis(methoxymethoxy)-4-bromo-2,6-dichlorotoluene 261958-52-5 C11H13BrCl2O4 360.032 4,6-二氯-5-甲基-1,3-苯二酚 4,6-dichloro-5-methylresorcinol 63992-61-0 C7H6Cl2O2 193.029 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-bromo-3,5-dichloro-2,6-dimethoxy-4-methylbenzene 1067073-73-7 C9H9BrCl2O2 299.979 —— 3,5-bis(methoxymethoxy)-4-bromo-2,6-dichlorotoluene 261958-52-5 C11H13BrCl2O4 360.032
反应信息
-
作为反应物:描述:2-bromo-4,6-dichloro-5-methylbenzene-1,3-diol 在 palladium on activated charcoal sodium chlorite 、 sodium dihydrogenphosphate 、 正丁基锂 、 2-甲基-2-丁烯 、 氢气 、 戴斯-马丁氧化剂 、 对甲苯磺酸 、 N,N-二异丙基乙胺 、 2,3-二氯-5,6-二氰基-1,4-苯醌 作用下, 以 四氢呋喃 、 甲醇 、 乙醚 、 乙醇 、 正己烷 、 二氯甲烷 、 叔丁醇 为溶剂, -78.0~20.0 ℃ 、101.33 kPa 条件下, 反应 13.17h, 生成 (+/-)-geodin参考文献:名称:甘丙肽受体亚型GalR1拮抗剂Sch 202596的合成研究:(±)-geodin(Sch 202596的螺香豆香酮部分)的有效合成摘要:以收敛的方式完成了与Sch 202596(1)的螺香古兰烷酮部分相对应的(±)-geodin [(±)-2 ]的高效合成。该合成方法的特征在于(i)芳基醛6与由芳基溴化物8原位产生的芳基锂7的偶联反应,以递送高度取代的二芳基甲醇24(6 + 7 → 24),以及ii)氧化的螺环化反应。二苯甲酮4构筑必要的螺香豆香烷骨架[ 4 →(±)-2 ]为关键步骤。芳香段图6和图8分别由可商购的3,5-二羟基苯甲酸甲酯(9)和5-甲基间苯二酚(10)制备。DOI:10.1016/s0040-4020(01)01250-9
-
作为产物:描述:3,5-二羟基甲苯 在 磺酰氯 、 溴 作用下, 以 二氯甲烷 、 氯仿 、 N,N-二甲基甲酰胺 为溶剂, 反应 4.5h, 生成 2-bromo-4,6-dichloro-5-methylbenzene-1,3-diol参考文献:名称:甘丙肽受体亚型GalR1拮抗剂Sch 202596的合成研究:(±)-geodin(Sch 202596的螺香豆香酮部分)的有效合成摘要:以收敛的方式完成了与Sch 202596(1)的螺香古兰烷酮部分相对应的(±)-geodin [(±)-2 ]的高效合成。该合成方法的特征在于(i)芳基醛6与由芳基溴化物8原位产生的芳基锂7的偶联反应,以递送高度取代的二芳基甲醇24(6 + 7 → 24),以及ii)氧化的螺环化反应。二苯甲酮4构筑必要的螺香豆香烷骨架[ 4 →(±)-2 ]为关键步骤。芳香段图6和图8分别由可商购的3,5-二羟基苯甲酸甲酯(9)和5-甲基间苯二酚(10)制备。DOI:10.1016/s0040-4020(01)01250-9
文献信息
-
Total Synthesis of the Marine Antibiotic Pestalone and its Surprisingly Facile Conversion into Pestalalactone and Pestalachloride A作者:Nikolay Slavov、Ján Cvengroš、Jörg-Martin Neudörfl、Hans-Günther SchmalzDOI:10.1002/anie.201003755日期:——Surprise, surprise! The total synthesis of the marine natural product pestalone (1), a highly substituted benzophenone with strong antibiotic acitivity, has provided insight into the surprising tendency of this and related molecules to undergo intramolecular Cannizzaro–Tishchenko‐type reactions. Pestalone can be readily converted into pestalachloride A, a strongly antifungal metabolite isolated from
-
Synthetic analogues of the antibiotic pestalone作者:Florian Kaiser、Hans-Günther SchmalzDOI:10.1016/s0040-4020(03)01136-0日期:2003.9The first synthetic study towards the natural product pestalone (1) is described culminating in the preparation of selected n-1 analogues. Pestalone is a chlorinated and prenylated benzophenone antibiotic, which is of interest due to a strong activity against methicillin-resistant staphylococcus aureus strains (MRSA). Key step of the synthesis is the nucleophilic addition of a highly functionalized aryllithium building block to a 2-prenylated 3,5-dialkoxy-benzaldehyde followed by oxidation. For the introduction of the prenyl sidechain by aryl-allyl coupling, different procedures were evaluated, among them the Stille reaction and a nickel pi-allyl complex coupling. (C) 2003 Elsevier Ltd. All rights reserved.
-
Studies toward the total synthesis of Sch 202596, an antagonist of the galanin receptor subtype GalR1: synthesis of geodin, the spirocoumaranone subunit of Sch 202596作者:Tadashi Katoh、Osamu OhmoriDOI:10.1016/s0040-4039(99)02005-5日期:2000.1An efficient synthesis of (+/-)-geodin [(+/-)-2] corresponding to the spirocoumaranone subunit of Sch 202596 (1) was accomplished in a convergent manner by utilizing coupling reaction of the aryl aldehyde 5 with the aryl bromide 6 and oxidative spirocyclization of the benzophenone 4 as the key steps. The aromatic segments 5 and 6 were prepared from commercially available methyl 3,5-dihydroxybenzoate (7) and 5-methylresorcinol (8), respectively. (C) 2000 Elsevier Science Ltd. All rights reserved.
表征谱图
-
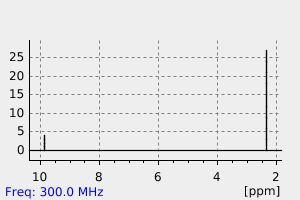
氢谱1HNMR
-
质谱MS
-
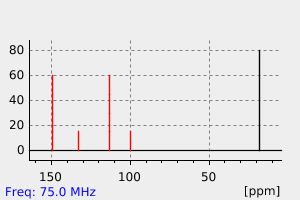
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫