2-戊硫醇 | 2084-19-7
中文名称
2-戊硫醇
中文别名
2-巯基戊烷;2-巯基戊烷(2-戊基硫醇);1-甲基丁硫醇;仲戊硫醇
英文名称
pentane-2-thiol
英文别名
2-pentanethiol;Pentanthiol-(2)
CAS
2084-19-7
化学式
C5H12S
mdl
MFCD00053516
分子量
104.216
InChiKey
QUSTYFNPKBDELJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-168.95°C
-
沸点:101 °C(lit.)
-
密度:0.827 g/mL at 25 °C(lit.)
-
闪点:80 °F
-
LogP:2.66
-
物理描述:colourless liquid
-
溶解度:slightly soluble in water; soluble in alcohol
-
折光率:1.442-1.452
-
保留指数:751
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:6
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:1
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:3.1
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R10
-
WGK Germany:3
-
危险品运输编号:UN 1993
-
海关编码:2930909090
-
包装等级:II
-
危险类别:3.1
SDS
制备方法与用途
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-methylbutyl methyl sulfide 13286-91-4 C6H14S 118.243
反应信息
-
作为反应物:参考文献:名称:Wagner; Reid, Journal of the American Chemical Society, 1931, vol. 53, p. 3410摘要:DOI:
-
作为产物:描述:参考文献:名称:Structure–Odor Correlations in Homologous Series of Alkanethiols and Attempts To Predict Odor Thresholds by 3D-QSAR Studies摘要:Homologous series of alkane-1-thiols, alkane-2-thiols, alkane-3-thiols, 2-methylalkane-1-thiols, 2-methylalkane-3-thiols, 2-methylalkane-2-thiols, and alkane-1,?-dithiols were synthesized to study the influence of structural changes on odor qualities and odor thresholds. In particular, the odor thresholds were strongly influenced by steric effects: In all homologous series a minimum was observed for thiols with five to seven carbon atoms, whereas increasing the chain length led to an exponential increase in the odor threshold. Tertiary alkanethiols revealed clearly lower odor thresholds than found for primary or secondary thiols, whereas neither a second mercapto group in the molecule nor an additional methyl substitution lowered the threshold. To investigate the impact of the SH group, odor thresholds and odor qualities of thiols were compared to those of the corresponding alcohols and (methylthio)alkanes. Replacement of the SH group by an OH group as well as S-methylation of the thiols significantly increased the odor thresholds. By using comparative molecular field analysis, a 3D quantitative structureactivity relationship model was created, which was able to simulate the odor thresholds of alkanethiols in good agreement with the experimental results. NMR and mass spectrometric data for 46 sulfur-containing compounds are additionally supplied.DOI:10.1021/jf506135c
文献信息
-
Activation and synthetic applications of thiostannanes. Efficient conversion of thiols into disulfides
-
Potassium tert-Butoxide Mediated Reductive C–P Cross-Coupling of Arylvinyl Sulfides through C–S Bond Cleavage作者:Qingle Zeng、Jie Feng、Qiaoling Zhang、Fuhai Li、Lu Yang、Ratnakar Reddy KuchukullaDOI:10.1055/s-0040-1707319日期:2021.1transition-metal-free t-BuOK-mediated reductive C–P cross-coupling reaction of arylvinyl sulfides with diarylphosphine oxides through C–S bond cleavage has been developed. This protocol not only permits the synthesis of diaryl(2-arylethyl)phosphine oxides, but also achieves an unprecedented construction of a C–P bond through C–S bond cleavage and reduction of a C–C double bond in one pot.
-
Chiral Thiols: The Assignment of Their Absolute Configuration by <sup>1</sup>H NMR作者:Silvia Porto、José Manuel Seco、Aurelio Ortiz、Emilio Quiñoá、Ricardo RigueraDOI:10.1021/ol7022196日期:2007.11.1A general NMR spectroscopy protocol for determination of absolute configuration of thiols, that includes the introduction of new aryl-tert-butoxyacetic acids as chiral derivatizing agents (CDAs), is described.
-
Carbon−Sulfur Bond Cleavage of Methyl-Substituted Thiophenes with Iridium(III)作者:Matthew R. Grochowski、William W. Brennessel、William D. JonesDOI:10.1021/om900114m日期:2009.5.11Reaction of [Cp*IrHCl]2 (Cp* = η5-C5Me5) with 2-methylthiophene and 2,5-dimethylthiophene at 120 °C in the presence of H2 results in the cleavage of the thiophene carbon−sulfur bond(s). In both cases the thiophenes are ring-opened and hydrogenated, resulting in dinuclear Ir complexes with bridging thiolates. The primary product in the reaction involving 2,5-dimethylthiophene is [Cp*IrCl]2(μ-H)(μ-S-2-hexyl)的[CP * IrHCl]的反应2(CP * =η 5 -C 5我5)与在H 2存在下甲基噻吩和2,5-二甲基噻在120℃ 2个在噻吩碳-硫裂解结果债券。在这两种情况下,噻吩都被开环和氢化,从而生成具有桥连硫醇盐的双核Ir配合物。涉及2,5-二甲基噻吩的反应中的主要产物是[CP * IrCl] 2(μ-H)(μ-S-2-己基)。该产品已经过鉴定,并以非对映异构体形式存在。在与2-甲基噻吩的反应中,产生了由五种产物组成的复杂混合物。产物分布由单取代和双取代的硫醇盐配合物组成,其中三种通过单晶X射线衍射进行了结构表征。这些产物均已独立合成,反应混合物的表征已通过1 H和13 C NMR光谱以及ESI-MS和元素分析完成。与2-乙酰基噻吩的反应显示出非常相似的反应性。X射线结构证实了存在的非对映异构体对的性质。
-
4-Dodecylbenzenesulfonic acid (DBSA) promoted solvent-free diversity-oriented synthesis of primary carbamates, S-thiocarbamates and ureas作者:Ali Reza Sardarian、Iman Dindarloo InalooDOI:10.1039/c5ra14528g日期:——
A simple and efficient solvent-free preparation of primary carbamates,
S -thiocarbamates and ureas from alcohols, phenols, thiols and amines in the presence of 4-dodecylbenzenesulfonic acid, as a cheap and green Brønsted acid, has been described.
表征谱图
-
氢谱1HNMR
-
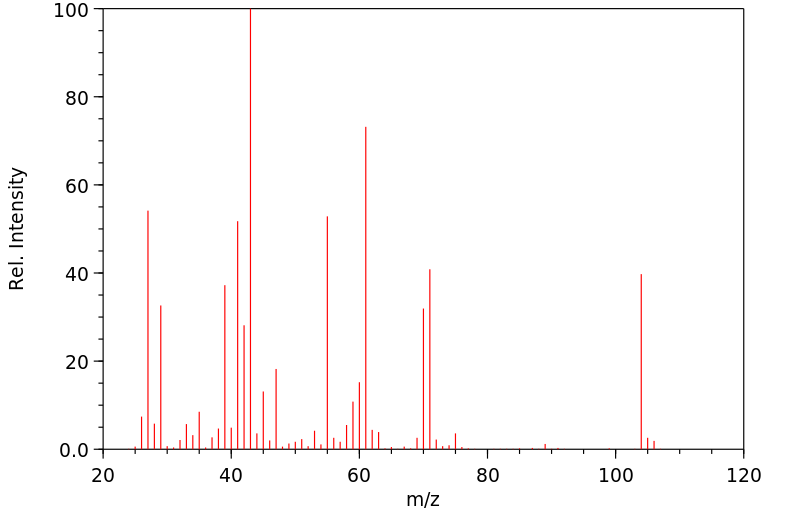
质谱MS
-
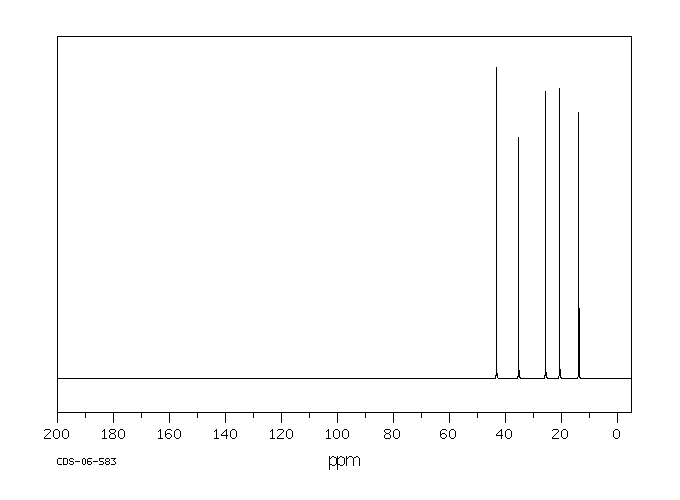
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
铜,丙烷-2-硫醇
铅,丙烷-1-硫醇
苏-(2R,4R)-戊二硫醇
羟基-乙醛
硫甘油
癸烷-2-硫醇
甲硫醇铅
甲硫醇钠
甲硫醇-d4
甲硫醇-S-d
甲硫醇
甲三硫醇
环辛硫醇
环戊硫醇
环戊基甲硫醇
环庚烷-1,1-二硫醇
环己硫醇
环己烷-1,1-二硫醇
环己基甲硫醇
环十二烷硫醇
环丙硫醇
环丙基甲硫醇
环丁硫醇
油烯基硫醇
氟甲硫醇
氘代甲硫醇-D3
正十四烷基硫醇
末端脱氧核苷酸转移酶
戊赤藓四硫醇
戊烷-3-硫醇
异戊硫醇
异戊烯基硫醇
异丙硫醇
异丁硫醇
庚-3-烯-4-硫醇
己-2-烯-1-硫醇
巯基甲烷-13C
巯基乙醛
巯基乙胺氢溴酸盐
巯基乙胺
巯基-十一胺盐酸盐
壬烷-2-硫醇
吡啶,2-(戊基硫代)-,1-氧化
叔壬基硫醇
叔十六硫醇
叔十二烷硫醇
叔丁基硫醇
反式-2-丁烯-1-硫醇
双(巯基环戊烷)四氯化钛
半胱胺盐酸盐