甘油三丁酸酯 | 60-01-5
中文名称
甘油三丁酸酯
中文别名
三丁酸甘油酯;甘油三正丁酸酯;三丁酸丙三醇酯;丁酸-1,2,3-三丙酯
英文名称
tributyrin
英文别名
glyceryl tributyrate;glycerol tributanoate;tributyrine;2,3-di(butanoyloxy)propyl butanoate
CAS
60-01-5
化学式
C15H26O6
mdl
——
分子量
302.368
InChiKey
UYXTWWCETRIEDR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-75 °C
-
沸点:305 °C
-
密度:1.0335
-
闪点:345 °F
-
溶解度:H2O:不溶
-
LogP:2.95
-
物理描述:Liquid
-
颜色/状态:COLORLESS
-
气味:FRUITY, BUTTERY
-
味道:Bitter taste
-
蒸汽压力:1.3X10-3 mm Hg at 25 °C /Estimated/
-
自燃温度:765 °F (407 °C)
-
分解:When heated to decomposition it emits acrid smoke and fumes.
-
折光率:Index of refraction = 1.4309 at 20 °C
-
保留指数:1812;1813;1815;1816
-
稳定性/保质期:
稳定存储,避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:21
-
可旋转键数:14
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:78.9
-
氢给体数:0
-
氢受体数:6
ADMET
代谢
...Tributyrinase.../是/一种专门用于水解tributyrins的酶。这种酶会被一些有机化合物抑制,尤其是选定的氟磷酸盐。...已经从大鼠脂肪组织中分离出一种对氟化物敏感的tributyrinase,它能够从1-单、1,2-二或tributyrin中释放出丁酸。
...Tributyrinase.../is/ an enzyme specific for the hydrolysis of tributyrins. This enzyme is inhibited by some organic compounds, particularly selected fluorophosphates. ...A fluoride-sensitive tributyrinase has been isolated from rat adipose tissue, which liberates butyric acid from 1-mono-, 1,2-di-, or tributyrin.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Porcine liver and kidney microsomes most actively hydrolyzed tributyrin. Liver microsomes from frogs, pigs, rats, cats, rabbits, guinea pigs, sheep, doves, dogs but not fish, also possessed esterase activity against tributyrin.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Studies of the hydrolysis of the glycerol fatty acid esters (/including/ tributyrin...) showed complete hydrolysis to glycerol and the corresponding fatty acids, butyric acid, 5-hydroxy-decanoic acid, and 5-hydroxydodecanoic acid, respectively.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
Effect of feeding tributyrin on toxicity of diisopropylfluorophosphate (DFP) in mice. After 36 hr of tributyrin feeding the DFP toxicity is 1.7 time normal & after 14 days of feeding it is only 1.15 times normal. .
来源:Hazardous Substances Data Bank (HSDB)
毒理性
人体暴露研究... 我们用递增剂量的tributyrin治疗了13名患者,剂量从50到400 mg/kg/天。剂量在隔夜禁食后在口服给药,每天一次,持续3周,然后休息1周。在两轮课程后没有出现大于2级的毒性,患者剂量递增。在第1天和第15天以及任何剂量递增后评估了血浆中丁酸盐的时间进程。3级毒性包括恶心、呕吐和肌肉痛。1级和2级毒性包括腹泻、头痛、腹部绞痛、恶心、贫血、便秘、氮质血症、头晕、疲劳、皮疹、脱发、异味、情绪低落和笨拙。tributyrin治疗没有一致的增加胎儿血红蛋白。
/HUMAN EXPOSURE STUDIES/ ... We treated 13 patients with escalating doses of tributyrin from 50 to 400 mg/kg/day. Doses were administered p.o. after an overnight fast, once daily for 3 weeks, followed by a 1-week rest. Intrapatient dose escalation occurred after two courses without toxicity greater than grade 2. The time course of butyrate in plasma was assessed on days 1 and 15 and after any dose escalation. Grade 3 toxicities consisted of nausea, vomiting, and myalgia. Grades 1 and 2 toxicities included diarrhea, headache, abdominal cramping, nausea, anemia, constipation, azotemia, lightheadedness, fatigue, rash, alopecia, odor, dysphoria, and clumsiness. There was no consistent increase in hemoglobin F with tributyrin treatment.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
实验室动物:急性暴露/ 吸入78 ppm达6小时在大鼠中引起了暂时的过度通气,但没有观察到死亡或其他症状。
/LABORATORY ANIMALS: Acute Exposure/ Inhalation of 78 ppm for 6 hr caused temporary hyperpnea in the rat, but no fatalities or other symptoms were observed.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
实验室动物:急性暴露/给小鼠喂食含有12%tributyrin的饮食,使丁酰胆碱酯酶活性增加到正常的约2.4倍,使乙酰胆碱酯酶活性降低到正常的约0.55倍。这些变化发生在饮食方案的第三天和第九天之间。
/LABORATORY ANIMALS: Acute Exposure/ Feeding mice a diet containing 12% tributyrin increased the butyrylcholinesterase activity to about 2.4 times normal & reduced the acetylcholinesterase activity to about 0.55 times normal. These changes intervene between 3rd & 9th day of regimen.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
实验室动物:急性暴露/ ... 在实验1中,通过瘤胃内给药84克或168克三丁酸甘油酯/给山羊/降低了葡萄糖。三丁酸甘油酯在给药后立即引起了短暂的血糖升高。β-羟基丁酸酯通过三丁酸甘油酯以剂量依赖性方式增加,导致血浆中胰岛素大量增加。在实验2中,通过瘤胃内给药84克三丁酸甘油酯降低了葡萄糖。
/LABORATORY ANIMALS: Acute Exposure/ ... In Experiment 1, glucose was decreased by intraruminal administration of 84 or 168 g of tributyrin /in goat/. Tributyrin caused transient hyperglycemia immediately after administration. beta-Hydroxybutyrate was increased in a dose-dependent manner by tributyrin, caused large increases of insulin in plasma. In Experiment 2, 84 g of tributyrin administered intraruminally decreased glucose
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
Tributyrin在大鼠的肠粘膜被吸收,在豚鼠则不会通过皮肤吸收。
Tributyrin is absorbed by the intestinal mucosa of the rat and is not absorbed cutaneously in guinea pigs.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
在人类中,每天一次口服注射50-400毫克/千克的tributyrin,持续3周,血浆中药物浓度峰值出现在给药后0.25-3小时。给予200毫克/千克剂量的人的血浆浓度峰值范围在0.1-0.45毫摩尔。在给予更高剂量的人中,并未观察到更高的血浆浓度。
In humans administered once daily oral injections of 50-400 mg/kg tributyrin for 3 weeks, peak plasma concentrations occurred 0.25-3 hr after dosing. The peak plasma concentration of those administered 200 mg/kg ranged from 0.1-0.45 mM. Higher plasma concentrations were not observed in those given higher doses.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
在小鼠和大鼠口服给予10.3克/千克的tributyrin后,血浆丁酸浓度分别在1.75和3.07毫摩尔时达到峰值。在小鼠中,它们在给药后10-60分钟内保持大于或等于1毫摩尔,而在大鼠中则是在30-90分钟内。
In mice and rats given 10.3 g/kg tributyrin orally, plasma butyrate concentrations peaked at 1.75 and 3.07 mM, respectively. They were >/= 1 mM from 10-60 min after dosing in mice and 30-90 min in rats.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
CD2F1雌性小鼠通过口服灌胃给予三丁酸甘油或通过静脉注射或口服灌胃给予丁酸钠。口服三丁酸甘油给小鼠的剂量为3.1、5.2、7.8和10.3 g/kg。静脉注射丁酸钠的剂量为0.31、0.62、0.94和1.25 g/kg。口服给予小鼠的丁酸钠剂量为5 g/kg。随后,在雌性Sprague-Dawley大鼠中进行了类似的研究。大鼠通过口服灌胃给予三丁酸甘油的剂量为3.6、5.2或10.3 g/kg,或静脉注射丁酸钠的剂量为500 mg/kg。通过气相色谱法测定血浆丁酸浓度。结果:在小鼠中,口服三丁酸甘油给药后,早在治疗后5分钟就检测到血浆丁酸浓度,并在给药后15至60分钟达到血浆丁酸浓度的峰值。随着三丁酸甘油剂量的增加,峰值血浆丁酸浓度成比例增加,但随着口服三丁酸甘油剂量的增加,血浆丁酸浓度与时间曲线下的面积(AUC)的增加超过了比例。当三丁酸甘油剂量为10.3 g/kg时,血浆丁酸浓度峰值约为1.75 mM,在给药后10至60分钟内保持在>1 mM。然而,大约10%的小鼠在接受这种剂量后急性死亡。当三丁酸甘油剂量为7.8 g/kg时,血浆丁酸浓度在给药后15分钟达到约1 mM,并在给药后60分钟内保持在0.8至1 mM之间。接受这种剂量的小鼠没有急性死亡。给予三丁酸甘油剂量为5.2和3.1 g/kg的小鼠在给药后45分钟达到的峰值血浆丁酸浓度分别约为0.9和0.5 mM。这些小鼠的血浆丁酸浓度在给药后120和90分钟内保持在0.1 mM以上。四种静脉注射丁酸钠的剂量也显示了非线性药物动力学,并且用一个具有可饱和消除的单室模型很好地描述。记录的米氏常数(Km)和过程的最大速度(Vmax)的值分别介于1.02至5.65 mM和0.60至1.82 mmol/min之间。记录的中央室体积(Vc)的值在0.48至0.72 l/kg之间变化。当静脉注射丁酸钠剂量为1.25 g/kg时,产生的峰值血浆丁酸浓度为10.5-17.7 mM,且血浆丁酸浓度在20-30分钟内保持在1 mM以上。口服给予小鼠5 g/kg的丁酸钠在给药后15分钟产生的峰值血浆丁酸浓度约为9 mM,且在给药后90分钟内血浆丁酸浓度超过1 mM。在大鼠中,10.3-g/kg的口服三丁酸甘油剂量在给药后75分钟产生的峰值血浆丁酸浓度约为3 mM,且丁酸浓度在给药后30至90分钟内超过1 mM。由5.2-和3.6-g/kg剂量在大鼠中产生的血浆丁酸浓度适当地低于10.3-g/kg剂量产生的浓度,并且没有非线性证据。500-mg/kg静脉注射丁酸钠在大鼠中产生的峰值血浆丁酸浓度约为11 mM,且随着给药后时间的推移,血浆丁酸浓度的下降与可饱和清除一致。
... Female CD2F1 mice were treated with tributyrin by oral gavage or with sodium butyrate by i.v. bolus or oral gavage. Oral tributyrin doses delivered to mice were 3.1, 5.2, 7.8, and 10.3 g/kg. Intravenous sodium butyrate doses were 0.31, 0.62, 0.94, and 1.25 g/kg. Oral sodium butyrate was given to mice at 5 g/kg. Subsequently, similar studies were performed in female Sprague-Dawley rats. Rats were given tributyrin by oral gavage at doses of 3.6, 5.2, or 10.3 g/kg or sodium butyrate i.v. at a dose of 500 mg/kg. Plasma butyrate concentrations were determined by gas chromatography. RESULTS: In mice, oral dosing with tributyrin resulted in detectable plasma butyrate concentrations as early as at 5 min after treatment and produced peak plasma butyrate concentrations at between 15 and 60 min after dosing. Peak plasma butyrate concentrations increased proportionally with increasing tributyrin dose, but as the oral tributyrin dose increased there was a greater than proportional increase in the area under the curve of plasma butyrate concentrations versus time (AUC). At a tributyrin dose of 10.3 g/kg, plasma butyrate concentrations peaked at approximately 1.75 mM and remained >1 mM for between 10 and 60 min after dosing. However, approximately 10% of mice treated with this dose died acutely. At a tributyrin dose of 7.8 g/kg, plasma butyrate concentrations reached approximately 1 mM by 15 min after dosing and remained between 0.8 and 1 mM until 60 min after dosing. No mouse treated with this dose died acutely. Mice given tributyrin doses of 5.2 and 3.1 g/kg achieved peak plasma butyrate concentrations of approximately 0.9 and 0.5 mM, respectively, by 45 min after dosing. Plasma butyrate concentrations in these mice remained above 0.1 mM until 120 and 90 min after dosing, respectively. The four i.v. doses of sodium butyrate resulted in plasma concentration-time profiles that also indicated nonlinear pharmacokinetics and were well described by a one-compartment model with saturable elimination. Values recorded for the Michaelis-Menten constant (Km) and the maximal velocity of the process (Vmax) ranged between 1.02 and 5.65 mM and 0.60 and 1.82 mmol/min, respectively. Values noted for the volume of the central compartment (Vc) varied between 0.48 and 0.72 l/kg. At 1.25 g/kg, i.v. sodium butyrate produced peak plasma butyrate concentrations of 10.5-17.7 mM, and plasma butyrate concentrations remained above 1 mM for 20-30 min. Sodium butyrate delivered orally to mice at 5 g/kg produced peak plasma butyrate concentrations of approximately 9 mM at 15 min after dosing and plasma butyrate concentrations exceeding 1 mM for 90 min after dosing. In rats the 10.3-g/kg oral dose of tributyrin produced peak plasma butyrate concentrations of approximately 3 mM by 75 min after dosing and butyrate concentrations exceeding 1 mM from 30 to 90 min after dosing. The plasma butyrate concentrations produced in rats by 5.2- and 3.6-g/kg doses were appropriately lower than those produced by the 10.3-g/kg dose, and there was no evidence of nonlinearity. The 500-mg/kg i.v. dose of sodium butyrate produced peak plasma butyrate concentrations in rats of approximately 11 mM, and the decline in plasma butyrate concentrations with time after dosing was consistent with saturable clearance.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
血浆丁酸盐浓度在0.25到3小时后达到峰值,随着剂量的增加而增加,范围从0到0.45毫摩尔。在三名剂量增加的患者中,峰值浓度没有增加。丁酸盐的药代动力学在第1天和第15天没有差异。因为达到了接近体外有效的血浆峰值浓度(0.5-1毫摩尔),但丁酸盐在给药后5小时从血浆中消失,所以我们现在正在研究每日三次给药的剂量递增,起始剂量为450毫克/千克/天。
... Peak plasma butyrate concentrations occurred between 0.25 and 3 h after dose, increased with dose, and ranged from 0 to 0.45 mM. Peak concentrations did not increase in three patients who had dose escalation. Butyrate pharmacokinetics were not different on days 1 and 15. Because peak plasma concentrations near those effective in vitro (0.5-1 mM) were achieved, but butyrate disappeared from plasma by 5 h after dose, we are now pursuing dose escalation with dosing three times daily, beginning at a dose of 450 mg/kg/day.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
安全说明:S24/25
-
WGK Germany:1
-
海关编码:2915900090
-
RTECS号:ET7350000
-
危险性防范说明:P233,P260,P261,P264,P271,P280,P302+P352,P304,P304+P340,P305+P351+P338,P312,P321,P332+P313,P337+P313,P340,P362,P403,P403+P233,P405,P501
-
危险性描述:H315,H319,H335
-
储存条件:本品应密封于阴凉处保存。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 三丁酸甘油酯;甘油三丁酸酯 |
| 化学品英文名称: | Glyceryl tributyrate;Tributyrin |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 60-01-5 |
| 分子式: | C 15 H 26 O 6 |
| 分子量: | 302.41 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | |||
| 化学品名称:三丁酸甘油酯;甘油三丁酸酯 | |||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 可能有刺激作用。一般认为,作为食品及工业接触中不存在卫生问题。遇热分解释出有刺激性的烟雾。 |
| 环境危害: | |
| 燃爆危险: |
| 第四部分:急救措施 |
| 皮肤接触: | 用肥皂水及清水彻底冲洗。就医。 |
| 眼睛接触: | 拉开眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 脱离现场至空气新鲜处。就医。 |
| 食入: | 误服者,饮适量温水,催吐。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇高热、明火或氧化剂,有引起燃烧的危险。 |
| 有害燃烧产物: | |
| 灭火方法及灭火剂: | 雾状水、抗溶性泡沫、二氧化碳、干粉。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | |
| 闪点(℃): | 100 |
| 自燃温度(℃): | |
| 爆炸下限[%(V/V)]: | |
| 爆炸上限[%(V/V)]: | |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 疏散泄漏污染区人员至安全区,禁止无关人员进入污染区,建议应急处理人员戴好口罩、护目镜,穿工作服。用大量水冲洗,经稀释的污水放入废水系统。如大量泄漏,利用围堤收容,然后收集、转移、回收或无害处理后废弃。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | |
| 储存注意事项: |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联 MAC:未制订标准美国TLV—TWA:未制订标准 |
| 监测方法: | |
| 工程控制: | 生产过程密闭,全面通风。 |
| 呼吸系统防护: | 应戴口罩。高浓度环境中,建议佩戴防毒面具。 |
| 眼睛防护: | 一般不需特殊防护。 |
| 身体防护: | 穿工作服。 |
| 手防护: | 戴防护手套。 |
| 其他防护: | 工作后,淋浴更衣。 |
| 第九部分:理化特性 |
| 外观与性状: | 无色油状液体,带有苦味。 |
| pH: | |
| 熔点(℃): | -75 |
| 沸点(℃): | 305~310 |
| 相对密度(水=1): | 1.0320 |
| 相对蒸气密度(空气=1): | |
| 饱和蒸气压(kPa): | |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | 100 |
| 引燃温度(℃): | |
| 爆炸上限%(V/V): | |
| 爆炸下限%(V/V): | |
| 分子式: | C 15 H 26 O 6 |
| 分子量: | 302.41 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 不溶于水,易溶于醇、醚、氯仿。 |
| 主要用途: | 用于制造食品、肥皂、蜡烛等,也用作溶剂和增塑剂。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:320mg/kg(小鼠静脉) LC50: |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | |
| UN编号: | |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 储存于阴凉、通风仓间内。远离火种、热源。保持容器密封。防潮、防晒。应与氧化剂分开存放。搬运时要轻装轻卸,防止包装及容器损坏。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 6 |
| MSDS修改日期: | 年月日 |
制备方法与用途
理化特性
甘油三丁酸酯是由一分子甘油和三分子丁酸经脂化反应制得,是一种短链脂肪酸酯。它不溶于水但易溶于乙醚和乙醇等有机溶剂,并具有较低的熔点(-75℃)和较高的沸点(305~310℃)。这种性质使其在高温、光照或制粒条件下理化性质基本保持不变,适合各类饲料加工工艺。
用途甘油三丁酸酯由一分子甘油与三分子丁酸反应生成。自然界中它存在于动物脂肪、植物油和奶制品等中。工业上主要用于人造黄油的生产,并作为饲料添加剂使用,在生物科技领域用作筛选和表征酯酶的底物。
饲料添加剂甘油三丁酸酯是一种由4个碳原子组成的短链脂肪酸酯,为无色近油状液体,略带脂肪香味。它可以顺利通过胃肠道并被缓慢分解成丁酸,在肠道后端发挥作用。研究表明,甘油三丁酸酯能促进肠道绒毛生长、增强营养物质消化、维持肠道菌群平衡、加强紧密连接、促进黏蛋白分泌和提高免疫力,从而提升动物的生产性能。
因此,将其添加到饲料中已成为改善动物肠道屏障功能、促进其生长的有效策略,并广泛应用于畜牧业养殖过程中。
含量分析甘油三丁酸酯的含量可通过两种方法测定:一种是按OT-18中的方法一进行测定时,取样量为1.5g,当量因子(e)设为50.40;另一种是使用气相色谱法(GT-10-4)中非极性柱的方法。
毒性甘油三丁酸酯被认定为GRAS物质(FEMA)。其急性毒性试验显示,大鼠经口摄入3200mg/kg后无致死效应。
使用限量- 软饮料:0.10 mg/kg
- 冷饮:0.04 mg/kg
- 糖果:0.33~1000 mg/kg
- 焙烤食品:290 mg/kg
- 布丁类:0.36 mg/kg
- 人造奶油:2.0~5.0 mg/kg
甘油三丁酸酯为白色近油状液体,几乎无气味但略带脂肪香气。其沸点为308℃,熔点为-75℃,易溶于乙醇、氯仿和乙醚,极难溶于水(0.010%)。天然存在于牛脂中。
用途 生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 丁酸甘油酯 1-monobutyrin 557-25-5 C7H14O4 162.186 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— β-dibutyrin 24814-35-5 C11H20O5 232.277 —— 1,2-dibutanoyl-sn-glycerol 30403-46-4 C11H20O5 232.277 —— 1,2-sn-dibutyroylglycerol 96739-06-9 C11H20O5 232.277 —— 3-Butyryl-1,2-diacetyl-sn-glycerin 57416-28-1 C11H18O6 246.26 2,3-二(1-氧代丁氧基)丙基硬脂酸酯 1,2-bis-butyryloxy-3-stearoyloxy-propane 56149-03-2 C29H54O6 498.744 三硬脂酸甘油酯 glycerol tristearate 555-43-1 C57H110O6 891.497 —— 2-butyryloxy-1,3-bis-stearoyloxy-propane 66411-63-0 C43H82O6 695.12 —— rac-1-Butyryl-2,3-distearoyl-glycerin 139665-43-3 C43H82O6 695.12 —— 1,3-dibutyryl-2-stearoylglycerol 139665-39-7 C29H54O6 498.744 三醋精 triacetylglycerol 102-76-1 C9H14O6 218.207 二十二碳六烯酸甘油三酯(CIS-4,7,10,13,16,19) tridocosahexaenoyl glycerol 11094-59-0 C69H98O6 1023.53 1,2,3-三(二十碳五烯酰基)甘油 trieicosapentaenoylglycerol 99660-94-3 C63H92O6 945.42 二丁酸甘油酯 1,3-dibutanoyloxy-2-propanol 17364-00-0 C11H20O5 232.277 2-单丁酰甘油 2-monobutyroylglycerol 70916-53-9 C7H14O4 162.186 丁酸甘油酯 1-monobutyrin 557-25-5 C7H14O4 162.186 —— 1-sn-monobutyroylglycerol 5309-42-2 C7H14O4 162.186 —— (S)-2,3-dihydroxypropyl butyrate 126254-87-3 C7H14O4 162.186 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:映射的使用比色筛选新的水解酶的底物选择性:从脂肪酶thermocatenulatus芽孢杆菌和蛇口壳piliferum,从酯酶荧光假单胞菌和链霉菌diastatochromogenes摘要:生物化学和分子生物学的最新进展简化了新型水解酶的发现和制备。尽管这些水解酶可能解决了有机合成中的问题,但测量其选择性(尤其是对映选择性)仍然繁琐且耗时。最近,我们开发了一种比色筛选方法来测量水解酶的对映选择性。在这里,我们应用这种快速筛选方法来绘制四种新型水解酶的底物选择性图:来自嗜热芽孢杆菌的脂肪酶(DSM 730,BTL2)和丝状真菌Ophiostoma piliferum(NRRL 18917,OPL)以及来自两种细菌荧光假单胞菌(Pseudomonas fluorescens( SIK-W1,酯酶I,PFE)和链霉菌(Tü20,SDE)。我们筛选了29种底物的通用文库和23对对映异构体的手性文库。所有四种水解酶均催化非天然底物的水解,但是与两种酯酶相比,两种脂肪酶接受的底物范围更广。不出所料,这两种脂肪酶偏爱疏水性更高的底物,而两种酯酶则偏爱较小的底物。确认了该酚醛酸酯的一些中等对映选择性反应:BTL2,丁酸酯,EDOI:10.1016/s0957-4166(01)00072-6
-
作为产物:参考文献:名称:氧化石墨催化羧酸与环氧的高效和区域选择性开环摘要:据报道,在无金属条件下,环氧化物与各种羧酸的有效,简单和区域选择性的开环反应。环氧化物的开环发生在作为有效和可用催化剂的氧化石墨存在下,以高收率生产相应的2-羟基单酯和1,2-二酯衍生物。亲核试剂的区域选择性攻击,较短的反应时间,无金属条件和催化剂的可重复使用性是本方案的优点。DOI:10.2174/1570178616666190401194252
-
作为试剂:描述:参考文献:名称:Vercellone, 1938, vol. 25, p. 207,208摘要:DOI:
文献信息
-
[EN] COMPOSITIONS AND METHODS FOR THE TREATMENT OF ATHEROTHROMBOSIS<br/>[FR] COMPOSITIONS ET PROCÉDÉS POUR LE TRAITEMENT DE L'ATHÉROTROMBOSE申请人:KANDULA MAHESH公开号:WO2013024376A1公开(公告)日:2013-02-21The disclosures herein provide compounds of formula I or its pharmaceutical acceptable salts, as well as polymorphs, enantiomers, stereoisomers, solvates, and hydrates thereof. These salts may be formulated as pharmaceutical compositions. The pharmaceutical compositions may be formulated for peroral administration- transdermal administration, transmucosal, syrups, topical, extended release, sustained release, or injection. Such compositions may foe used to treatment of vascular disorders or conditions such as thrombotic cerebrovascular or cardiovascular disease or its associated complications.本公开提供公式I的化合物或其药用可接受的盐,以及其多晶型、对映体、立体异构体、溶剂合物和水合物。这些盐可以制成药物组合物。药物组合物可以用于口服、经皮、经粘膜、糖浆、局部、延长释放、持续释放或注射的给药。这种组合物可用于治疗血管疾病或病况,如血栓性脑血管或心血管疾病或其相关并发症。
-
[EN] PHOTOCHROMIC AND ELECTROCHROMIC DIARYLETHENE COMPOUNDS WITH IMPROVED PHOTOSTABILITY AND SOLUBILITY<br/>[FR] COMPOSÉS DIARYLÉTHÈNE PHOTOCHROMIQUES ET ÉLECTROCHROMES PRÉSENTANT UNE PHOTOSTABILITÉ ET UNE SOLUBILITÉ AMÉLIORÉES申请人:SWITCH MAT INC公开号:WO2020198868A1公开(公告)日:2020-10-08A diarylethene compound reversibly convertible under photochromic and electrochromic conditions between a ring-open isomer of Formula (1A) and a ring-closed isomer of Formula (IB) wherein R5 is a substituted phenyl ring and Re is a substituted thiophene ring is provided. The photochromic-electrochromic diarylethene compound of Formula (1A)/(1B) have improved photochromic, electrochromic or photochromic and electrochromic properties, and is useful to provide variation of the light transmission properties of optical filters. The compound also possesses improved solubility making it suitable for incorporation in commercial products..
-
NOVEL IMMUNOMODULATOR AND ANTI-INFLAMMATORY COMPOUNDS申请人:MUTHUPPALANIAPPAN Meyyappan公开号:US20110275603A1公开(公告)日:2011-11-10The present invention provides dihydroorotate dehydrogenase inhibitors, methods of preparing them, pharmaceutical compositions containing them and methods of treatment, prevention and/or amelioration of diseases or disorders wherein the inhibition of Dihydroorotate dehydrogenase is known to show beneficial effect.
-
[EN] (HETERO)ARYL CYCLOPROPYLAMINE COMPOUNDS AS LSD1 INHIBITORS<br/>[FR] COMPOSÉS D'(HÉTÉRO)ARYL-CYCLOPROPYLAMINE À TITRE D'INHIBITEURS DE LSD1申请人:ORYZON GENOMICS SA公开号:WO2013057322A1公开(公告)日:2013-04-25The invention relates to (hetero)aryl cyclopropylamine compounds, including particularly the compounds of formula (I) as described and defined herein, and their use in therapy, including, e.g., in the treatment or prevention of cancer, a neurological disease or condition, or a viral infection.本发明涉及(杂)芳基环丙胺化合物,特别是如本文所述和定义的公式(I)的化合物,以及它们在治疗中的应用,例如,在治疗或预防癌症、神经系统疾病或状况、或病毒感染方面的应用。
-
[EN] (HETERO)ARYL CYCLOPROPYLAMINE COMPOUNDS AS LSD1 INHIBITORS<br/>[FR] COMPOSÉS (HÉTÉRO)ARYLE CYCLOPROPYLAMINES EN TANT QU'INHIBITEURS DE LSD1申请人:ORYZON GENOMICS SA公开号:WO2013057320A1公开(公告)日:2013-04-25The invention relates to (hetero)aryl cyclopropylamine compounds, including particularly the compounds of formula I as described and defined herein, and their use in therapy, including, e.g., in the treatment or prevention of cancer, a neurological disease or condition, or a viral infection.本发明涉及(杂)芳基环丙胺化合物,特别是如本文所述和定义的公式I的化合物,以及它们在治疗中的用途,例如,在治疗或预防癌症、神经疾病或状况、或病毒感染中的用途。
表征谱图
-
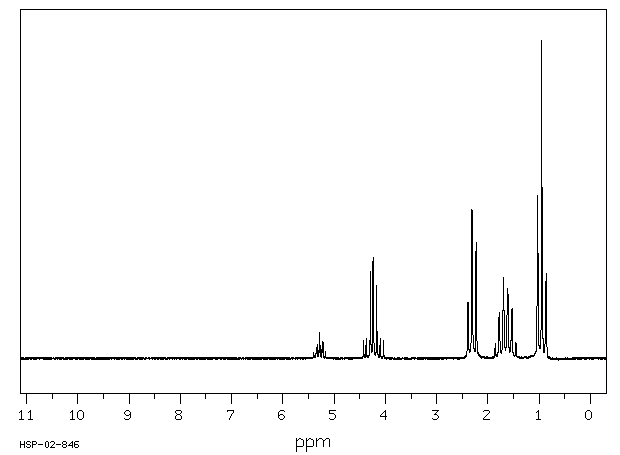
氢谱1HNMR
-
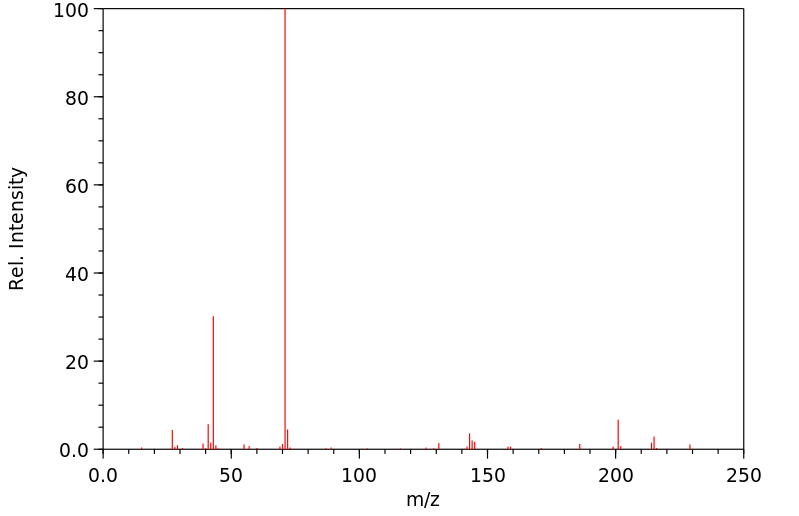
质谱MS
-
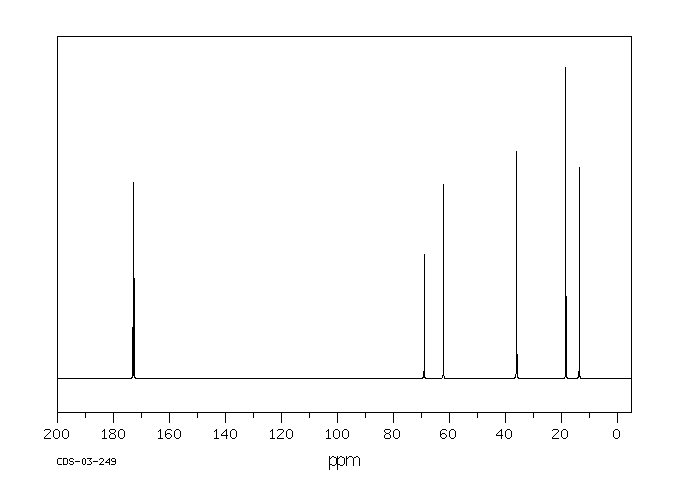
碳谱13CNMR
-
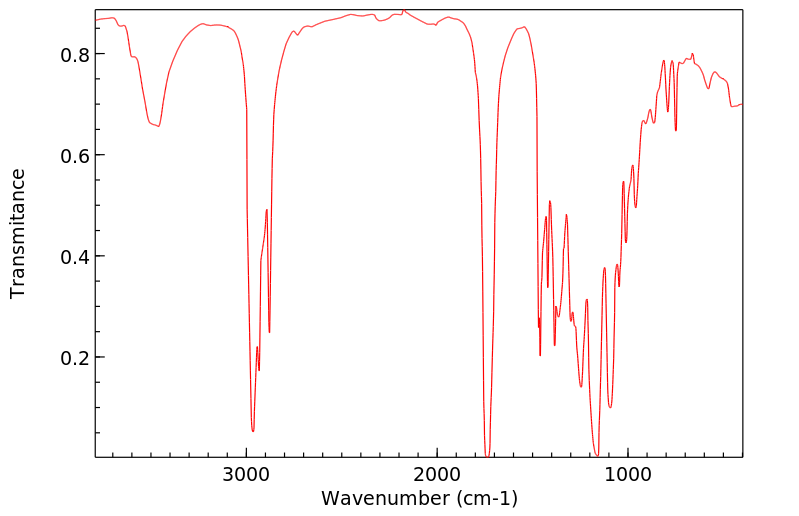
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-烯丙氧基-1,2-丙二醇
鲸蜡氧丙基甘油基甲氧丙基肉豆蔻酰胺
鲨肝醇硬脂酸酯
鲨肝醇
鲛肝醇
鲛肝醇
马来酸蓖麻油酯
辛氧基甘油
辛、癸酸甘油酯
甘油单肉豆寇酸酯
蓖麻油
苯丁酸甘油酯
苄氧基甲基-24-冠-8
肉豆蔻脑酸甘油酯
聚甘油基-2三异硬脂酸盐
聚甘油-6二硬脂酸酯
聚甘油-6三硬脂酸酯
聚甘油-4硬脂酸酯
聚甘油-3癸酸酯
聚甘油-3油酸酯
聚甘油-3二油酸酯
聚甘油-2硬脂酸酯
聚甘油-2油醚
聚甘油-2油酸酯
聚甘油-2月桂酸酯
聚甘油-2异硬脂酸酯
聚甘油-2倍半辛酸酯
聚甘油-2二油酸酯
聚甘油-2 癸酸酯
聚甘油-10油酸酯
聚甘油-10五硬脂酸酯
聚甘油-10二油酸酯
缩水甘油醚
神经酸甘油单酯
碳酸二(1-异丙氧基甲基-2-异丙氧乙基)酯
甲基3-[3-(叔-丁氧基羰基氨基)吖丁啶-1-基]-2-甲基-丙酸酯
甘油褐煤酸酯
甘油葡糖苷
甘油花生酸酯
甘油花生四烯酸酯
甘油芥酸酯
甘油聚醚-7三乙酸酯
甘油聚醚-26
甘油硫代乙醇酸酯
甘油烯丙基醚
甘油棕榈酸酯
甘油庚酸酯
甘油巯基丙酸酯
甘油基-1,2-二亚油酸酯-3-油酸酯
甘油基-1,2,3-三乙酰乙酸酯