2-甲基-2,3-戊二醇 | 7795-80-4
中文名称
2-甲基-2,3-戊二醇
中文别名
——
英文名称
2-methylpentane-2,3-diol
英文别名
2-methyl-2,3-pentanediol;2-methyl 2,3-pentandiol;2-Methyl-pentan-2,3-diol
CAS
7795-80-4
化学式
C6H14O2
mdl
——
分子量
118.176
InChiKey
GSQFUEPQVUSAPE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:26.38°C (estimate)
-
沸点:221.7°C (rough estimate)
-
密度:0.9843 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Diethoxytriphenylphosphorane: a mild, regioselective cyclodehydrating reagent for conversion of diols to cyclic ethers: stereochemistry, synthetic utility, and scope摘要:DOI:10.1021/ja00304a030
-
作为产物:描述:参考文献:名称:Fritz,H.P.; Wuerminghausen,T., Zeitschrift fur Naturforschung, Teil B: Anorganische Chemie, Organische Chemie, 1977, vol. 32, p. 241 - 248摘要:DOI:
文献信息
-
Manganese catalyzed cis-dihydroxylation of electron deficient alkenes with H2O2作者:Pattama Saisaha、Dirk Pijper、Ruben P. van Summeren、Rob Hoen、Christian Smit、Johannes W. de Boer、Ronald Hage、Paul L. Alsters、Ben L. Feringa、Wesley R. BrowneDOI:10.1039/c0ob00102c日期:——A practical method for the multigram scale selective cis-dihydroxylation of electron deficient alkenes such as diethyl fumarate and N-alkyl and N-aryl-maleimides using H2O2 is described. High turnovers (>1000) can be achieved with this efficient manganese based catalyst system, prepared in situ from a manganese salt, pyridine-2-carboxylic acid, a ketone and a base, under ambient conditions. Under optimized conditions, for diethyl fumarate at least 1000 turnovers could be achieved with only 1.5 equiv. of H2O2 with d/l-diethyl tartrate (cis-diol product) as the sole product. For electron rich alkenes, such as cis-cyclooctene, this catalyst provides for efficient epoxidation.
-
Regioselective phosphoranylation and cyclodehydration of triols with diethoxytriphenylphosphorane作者:Jeffery W. Kelly、Slayton A. EvansDOI:10.1021/ja00284a036日期:1986.11The structures of the 2,2,2-triphenyl-1,3,2-dioxaphospholanes are readily assessed from /sup n/J/sub /sup 31/P-/sup 13/C/ (n = 2,3) coupling constants and /sup 31/P chemical shifts. Alkyl and aryl substituents attached to the dioxaphospholane ring also induce pronounced substituent shielding effects on the /sup 31/P resonance of the phospholanes. These effects are useful in corroborating the structural二乙氧基三苯基膦 (DTPP) 选择性地二膦酰化 1,2,4-三醇中的邻二醇官能团,提供热力学稳定的 2,2,2-三苯基-1,3,2-二氧杂膦。当经受热解条件时,这些二氧杂磷杂环戊烷分解形成瞬态甜菜碱,随后通过三苯基氧化膦的 3-exo-tet 挤出而坍塌成环氧化物。2,2,2-triphenyl-1,3,2-dioxaphospholanes 的结构很容易从 /sup n/J/sub /sup 31/P-/sup 13/C/ (n = 2,3) 耦合评估常数和 /sup 31/P 化学位移。连接到二氧杂磷杂环戊烷环上的烷基和芳基取代基也会对磷杂环戊烷的 /sup 31/P 共振产生显着的取代基屏蔽效应。这些效应有助于证实二氧正膦的结构归属。
-
Osmium-Catalyzed Dihydroxylation of Olefins Using Dioxygen or Air as the Terminal Oxidant作者:Christian Döbler、Gerald M. Mehltretter、Uta Sundermeier、Matthias BellerDOI:10.1021/ja000802u日期:2000.10.1The osmium-catalyzed dihydroxylation of various olefins using molecular oxygen or air as the stoichiometric oxidant is reported. Aromatic olefins yield the corresponding diols in good to excellent chemoselectivities under optimized pH conditions (pH = 10.4−12.0). Air can be used under moderate pressures (3−9 bar) instead of dioxygen as the reoxidant. By increasing the oxygen content of the solution
-
Photochemical reaction of alcohols-I
-
Shape selective oxidation using titanium silicates: epoxidation of dihydromyrcene and the model compounds 2-methylpent-2-ene and 3-methylpent-1-ene作者:Lee J. Schofield、Owain J. Kerton、Paul McMorn、Donald Bethell、Simon Ellwood、Graham J. HutchingsDOI:10.1039/b207425g日期:2002.12.6The regioselective epoxidation of dihydromyrcene has been studied in the presence of the titanium-containing silicates TS-1 and TiAlβ using aqueous hydrogen peroxide, tert-butyl hydroperoxide and urea–hydrogen peroxide as oxidants. Epoxides were observed with TS-1 and aqueous hydrogen peroxide, and with TiAlβ when used in conjunction with the urea–hydrogen peroxide complex and tert-butyl hydroperoxide. Epoxidation occurs exclusively at the more electron-rich double bond in the presence of both catalysts. The epoxidation of dihydromyrcene has also been studied under triphasic conditions (two immiscible liquid phases and one catalyst phase) rather than biphasic conditions (one liquid phase and one catalyst phase). The alcoholysis reaction of the resulting epoxide was found to proceed via the more stabilised cation intermediate under biphasic conditions. In contrast, alcoholysis under triphasic conditions proceeded to form both the favoured (major) and unfavoured (minor) ether alcohols in ratios up to 2 ∶ 1. Model compounds, (2-methylpent-2-ene and 3-methylpent-1-ene) which simulate the electronic environment around each of the double bonds in dihydromyrcene, have been used to study the degree of epoxidation of each double bond separately and under competitive conditions. When the model substrates are studied separately, the rate of epoxidation of the two double bonds are comparable. When the model substrates are epoxidised in a competitive manner, the electron-deficient double bond is oxidised in preference which is different to that observed for dihydromyrcene.二氢美克烯的选择性环氧化在含钛的硅酸盐TS-1和TiAlβ的存在下进行了研究,使用的氧化剂包括水合过氧化氢、叔丁基过氧化氢和尿素-过氧化氢复合物。在TS-1和水合过氧化氢的体系中观察到了环氧化物,在TiAlβ的体系中则需与尿素-过氧化氢复合物和叔丁基过氧化氢共同使用。环氧化反应仅在更富电子的双键上进行,在两种催化剂的存在下均为如此。二氢美克烯的环氧化反应还在三相条件下(两个不混溶的液相和一个催化剂相)进行了研究,而非在两相条件下(一个液相和一个催化剂相)。结果发现,在两相条件下生成的环氧化物的醇解反应通过更稳定的阳离子中间体进行。相比之下,在三相条件下的醇解反应则同时形成了主要( favored)和次要(unfavored)的醚醇,其比例可达2:1。为了研究每个双键的环氧化程度,使用了模拟二氢美克烯中每个双键周围电子环境的模型化合物(2-甲基戊-2-烯和3-甲基戊-1-烯),在竞争条件下分别进行了单独研究。当单独研究这些模型底物时,两个双键的环氧化速率相当。然而,在竞争性环氧化中,电子不足的双键优先被氧化,这与二氢美克烯的观察结果有所不同。
表征谱图
-
氢谱1HNMR
-
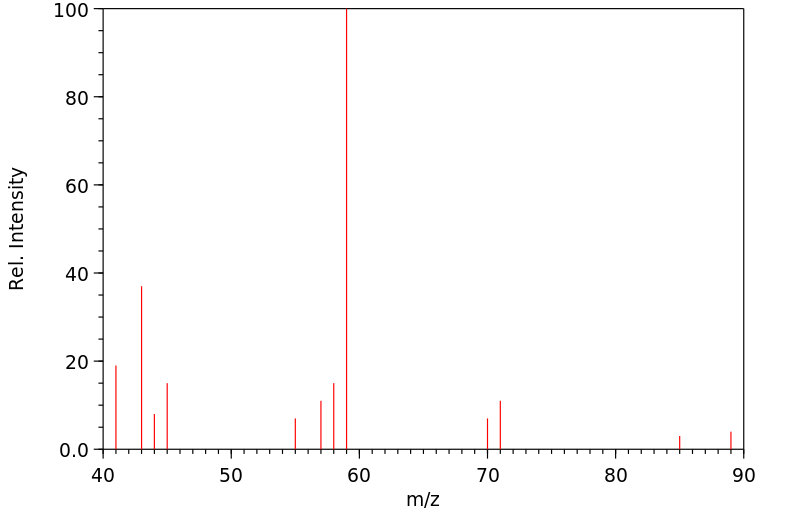
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷