2-甲基-3-(2-甲基丙基)吡嗪 | 13925-06-9
中文名称
2-甲基-3-(2-甲基丙基)吡嗪
中文别名
2-异丁基-3-甲基吡嗪;2-甲基-3-异丁基吡嗪
英文名称
2-methyl-3-(2-methylpropyl)pyrazine
英文别名
2-isobutyl-3-methyl pyrazine;2-isobutyl-3-methylpyrazine;2-methyl-3-isobutylpyrazine;2-isobutyl-3-methyl-pyrazine;2-Isobutyl-3-methyl-pyrazin;2-Methyl-3-isobutyl-pyrazin
CAS
13925-06-9
化学式
C9H14N2
mdl
MFCD00059770
分子量
150.224
InChiKey
ZHMIODDNZRIENW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:74 °C
-
沸点:199 °C(lit.)
-
密度:0.942 g/mL at 25 °C(lit.)
-
闪点:180 °F
-
LogP:1.96
-
物理描述:colourless to slightly yellow liquid with a green, earthy, celery odour
-
溶解度:soluble in water, oils, organic solvents
-
折光率:1.488-1.498
-
保留指数:1114;1134;1144
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.555
-
拓扑面积:25.8
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:2933990090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
SDS
2-异丁基-3-甲基吡嗪 修改号码:5
模块 1. 化学品
产品名称: 2-Isobutyl-3-methylpyrazine
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 可燃液体
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离明火/热表面。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
2-异丁基-3-甲基吡嗪 修改号码:5
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-异丁基-3-甲基吡嗪
百分比: >98.0%(GC)
CAS编码: 13925-06-9
俗名: 2-Methyl-3-isobutylpyrazine
分子式: C9H14N2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
2-异丁基-3-甲基吡嗪 修改号码:5
模块 8. 接触控制和个体防护
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 极淡的黄色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 92 °C/4kPa
闪点: 82°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.94
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
2-异丁基-3-甲基吡嗪 修改号码:5
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2-Isobutyl-3-methylpyrazine
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 可燃液体
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离明火/热表面。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
2-异丁基-3-甲基吡嗪 修改号码:5
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-异丁基-3-甲基吡嗪
百分比: >98.0%(GC)
CAS编码: 13925-06-9
俗名: 2-Methyl-3-isobutylpyrazine
分子式: C9H14N2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
2-异丁基-3-甲基吡嗪 修改号码:5
模块 8. 接触控制和个体防护
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 极淡的黄色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 92 °C/4kPa
闪点: 82°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.94
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
2-异丁基-3-甲基吡嗪 修改号码:5
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-Isobutyl-3-methylchinoxalin 25058-21-3 C13H16N2 200.283
反应信息
-
作为反应物:描述:2-甲基-3-(2-甲基丙基)吡嗪 在 苯甲醛 作用下, 以2.5 g (48.8%)的产率得到2-(2-hydroxy-2-phenylethyl)-3-isobutylpyrazine参考文献:名称:Flavorant-release additives and compositions containing them摘要:这项发明提供了含有一种新型杂芳香味释放添加剂的吸烟组合物,其结构如下:其中R是氢或C₁-C₄烷基;R¹是氢或C₁-C₈烷基;R²是C₃-C₁₂芳香取代基;但至少有两个R¹基团是C₁-C₈烷基基团。在典型的香烟吸烟条件下,2-异丙基吡嗪和苯乙酮作为热解产物释放出来,增强了主流烟和旁通烟的风味和香气。公开号:EP0309203A3
-
作为产物:描述:5-Isobutyl-6-methyl-pyrazine-2,3-dicarboxylic acid 以 溶剂黄146 为溶剂, 反应 1.0h, 生成 2-甲基-3-(2-甲基丙基)吡嗪参考文献:名称:Heyns, Kurt; Behse, Ernst; Francke, Wittko, Chemische Berichte, 1981, vol. 114, # 1, p. 240 - 245摘要:DOI:
文献信息
-
BITTER TASTE MODIFIERS INCLUDING SUBSTITUTED 1-BENZYL-3-(1-(ISOXAZOL-4-YLMETHYL)-1H-PYRAZOL-4-YL)IMIDAZOLIDINE-2,4-DIONES AND COMPOSITIONS THEREOF申请人:SENOMYX, INC.公开号:US20160376263A1公开(公告)日:2016-12-29The present invention includes compounds and compositions known to modify the perception of bitter taste, and combinations of said compositions and compounds with additional compositions, compounds, and products. Exemplary compositions comprise one or more of the following: cooling agents; inactive drug ingredients; active pharmaceutical ingredients; food additives or foodstuffs; flavorants, or flavor enhancers; food or beverage products; bitter compounds; sweeteners; bitterants; sour flavorants; salty flavorants; umami flavorants; plant or animal products; compounds known to be used in pet care products; compounds known to be used in personal care products; compounds known to be used in home products; pharmaceutical preparations; topical preparations; cannabis-derived or cannabis-related products; compounds known to be used in oral care products; beverages; scents, perfumes, or odorants; compounds known to be used in consumer products; silicone compounds; abrasives; surfactants; warming agents; smoking articles; fats, oils, or emulsions; and/or probiotic bacteria or supplements.本发明涵盖已知用于改变苦味感知的化合物和组合物,以及所述组合物和化合物与额外的组合物、化合物和产品的组合。示例组合物包括以下一种或多种:冷却剂;无活性药物成分;活性药用成分;食品添加剂或食品;调味剂或调味增强剂;食品或饮料产品;苦味化合物;甜味剂;苦味剂;酸味调味剂;咸味调味剂;鲜味调味剂;植物或动物产品;已知用于宠物护理产品中的化合物;已知用于个人护理产品中的化合物;已知用于家用产品中的化合物;制药制剂;局部制剂;大麻衍生或与大麻相关的产品;已知用于口腔护理产品中的化合物;饮料;香味、香水或除臭剂;已知用于消费品中的化合物;硅化合物;磨料;表面活性剂;发热剂;吸烟物品;脂肪、油脂或乳化剂;和/或益生菌或补充剂。
-
COMPOUNDS USEFUL AS MODULATORS OF TRPM8申请人:Senomyx, Inc.公开号:US20170096418A1公开(公告)日:2017-04-06The present disclosure relates to compounds which are useful as cooling sensation compounds.本公开涉及作为冷感化合物有用的化合物。
-
Synthesis and pyrolysis of two flavor precursors of oct-1-en-3-yl methylpyrazinecarboxylates作者:Miao Lai、Xiaoming Ji、Tao Tao、Yuanyuan Shan、Pengfei Liu、Mingqin ZhaoDOI:10.1007/s10973-016-6083-5日期:2017.6To rich flavor additive species of pyrazines, two new compounds of 3,6-dimethyl-2,5-pyrazinedicarboxylic acid 1-octen-3-yl ester (DMPOE) and 3,5,6-trimethyl-2-pyrazinecarboxylic acid 1-octen-3-yl ester (TMPOE) were synthesized by KMnO4 oxidation, acylating chlorination and esterification reaction, in which tetramethylpyrazine and 1-octen-3-ol were used as initial materials. Thermogravimetry (TG), differential scanning calorimeter (DSC) and pyrolysis–gas chromatography/mass spectrometry (Py–GC/MS) were conducted to investigate the thermal degradation behaviors of DMPOE and TMPOE. TG–DTG results indicated that the T p of DMPOE and TMPOE with the largest mass loss rate was at 310 and 250 °C, respectively. The T peak of DMPOE and TMPOE showed by DSC curves was 301.8 and 260.0 °C, respectively. Py–GC/MS was performed to benefit the simulation of cigarette burning conditions, and the results indicated that DMPOE and TMPOE could release specific flavors of 1-octen-3-ol and diversified alkylpyrazines. Furthermore, the thermal degradation mechanisms of the flavor precursors of DMPOE and TMPOE were discussed. The study on the thermal behavior of these two methylpyrazinecarboxylates would provide theoretical basis for their application in tobacco.为了丰富吡嗪类香料添加剂的种类,通过 KMnO4 氧化、酰化氯化和酰化氯化反应合成了 3,6-二甲基-2,5-吡嗪二羧酸 1-辛烯-3-基酯(DMPOE)和 3,5,6-三甲基-2-吡嗪二羧酸 1-辛烯-3-基酯(TMPOE)两种新化合物、以四甲基吡嗪和 1-辛烯-3-醇为初始原料,通过 KMnO4 氧化、酰化氯化和酯化反应合成了 3,6-二甲基-2,5-吡嗪二羧酸 1-辛烯-3-基酯(DMPOE)和 3,5,6-三甲基-2-吡嗪二羧酸 1-辛烯-3-基酯(TMPOE)。采用热重法(TG)、差示扫描量热仪(DSC)和热解-气相色谱/质谱法(Py-GC/MS)研究了 DMPOE 和 TMPOE 的热降解行为。TG-DTG结果表明,质量损失率最大的DMPOE和TMPOE的T峰分别位于310和250 ℃。DSC 曲线显示 DMPOE 和 TMPOE 的 T 峰分别为 301.8 和 260.0 ℃。为了模拟香烟燃烧条件,研究人员进行了 Py-GC/MS,结果表明 DMPOE 和 TMPOE 能释放出特定的 1-辛烯-3-醇和多种烷基吡嗪。此外,还讨论了 DMPOE 和 TMPOE 香味前体的热降解机制。对这两种甲基吡嗪羧酸盐热行为的研究将为它们在烟草中的应用提供理论依据。
-
NOVEL PYRROLIDINE DERIVED BETA 3 ADRENERGIC RECEPTOR AGONISTS申请人:Edmondson Scott D.公开号:US20120225886A1公开(公告)日:2012-09-06The present invention provides compounds of Formula (I), pharmaceutical compositions thereof, and method of using the same in the treatment or prevention of diseases mediated by the activation of β3-adrenoceptor.本发明提供了公式(I)的化合物,其药物组合物,以及使用它们治疗或预防通过激活β3肾上腺素受体介导的疾病的方法。
-
ENERGETIC COCRYSTALS FOR TREATMENT OF A SUBTERRANEAN FORMATION申请人:HALLIBURTON ENERGY SERVICES, INC.公开号:US20160177698A1公开(公告)日:2016-06-23The present invention relates to energetic cocrystals, and to methods for using the same for treatment of a subterranean formation. In various embodiments, the present invention provides a method of treating a subterranean formation, the method including obtaining or providing a composition including energetic cocrystals. Each energetic cocrystal independently includes an energetic compound and a secondary material. The method also includes placing the composition in a subterranean formation.本发明涉及具有能量共晶体的相关技术,以及使用这些技术治疗地下地层的方法。在各种实施例中,本发明提供了一种治疗地下地层的方法,该方法包括获得或提供包括能量共晶体的组合物。每个能量共晶体独立地包括一个能量化合物和一个辅助材料。该方法还包括将该组合物放置在地下地层中。
表征谱图
-
氢谱1HNMR
-
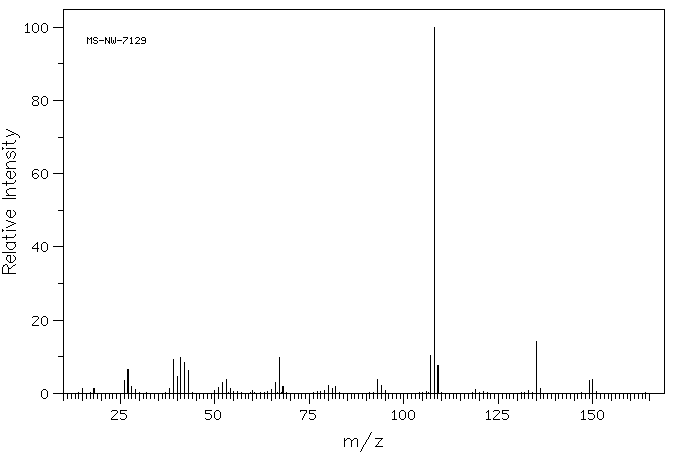
质谱MS
-
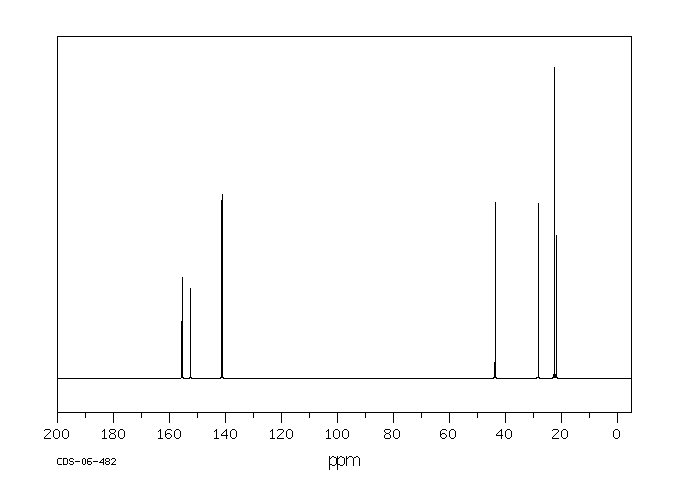
碳谱13CNMR
-
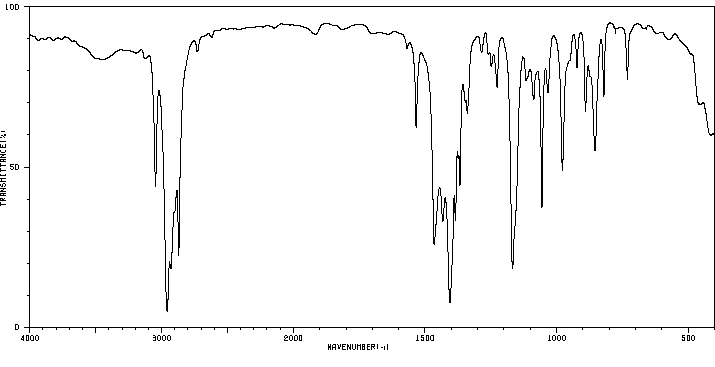
红外IR
-
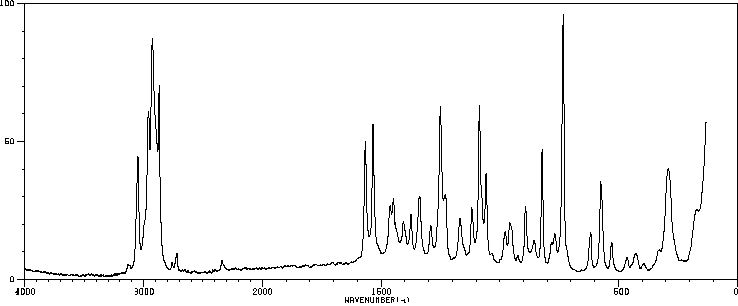
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3