3,3,4,4-四氟丁烷-2-醇 | 17425-25-1
中文名称
3,3,4,4-四氟丁烷-2-醇
中文别名
——
英文名称
3,3,4,4-tetrafluorobutan-2-ol
英文别名
3,3,4,4-Tetrafluor-butan-1-ol;3,3,4,4-Tetrafluor-butan-2-ol;2,2,3,3-tetrafluoro-1-methylpropanol;2-Butanol, 3,3,4,4-tetrafluoro-
CAS
17425-25-1
化学式
C4H6F4O
mdl
MFCD00021874
分子量
146.085
InChiKey
UPMGUZUMWYWMKI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:109-110 °C
-
密度:1.274±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:9
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:5
安全信息
-
海关编码:2905590090
反应信息
-
作为产物:描述:参考文献:名称:Promising prospects for using partially fluorinated alcohols as O-nucleophilic reagents in organofluoric synthesis摘要:Perfluoroolefins react with isopropyl alcohol under the conditions of radical initiation to form partially fluorinated aliphatic alcohols. The reactions of these alcohols with hexafluoropropylene and compounds containing labile halogen atoms (in particular, with allyl bromide and epichlorohydrin) were studied.DOI:10.1134/s1070427207030123
文献信息
-
Photochemical synthesis of fluoroalkanols based on tetrafluoroethylene作者:O. Paleta、V. Dědek、H. Reutschek、H.-J. TimpeDOI:10.1016/s0022-1139(00)83928-7日期:1989.3The conditions of the photochemical synthesis of fluoroalkanols H(C2F4)nC(OH)R1R2 (n = 1, 2) by the reaction of tetrafluoroethylene with alcohols in the presence of photoinitiators and sensitisers are described. The reaction is initiated by UV radiation and is performed under atmospheric pressure. The yields of alkanols were 0.1-0.39 mol using 250 W UV lamp after 6-8 hours and 73-82 % relatively to
-
Fluorine-containing alcohols and process for preparing the same申请人:DU PONT公开号:US02559628A1公开(公告)日:1951-07-10
-
PALETA, O.;DEDEK, V.;REUTSCHEK, H.;TIMPE, H. -J., J. FLUOR. CHEM., 42,(1989) N, C. 345-353作者:PALETA, O.、DEDEK, V.、REUTSCHEK, H.、TIMPE, H. -J.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
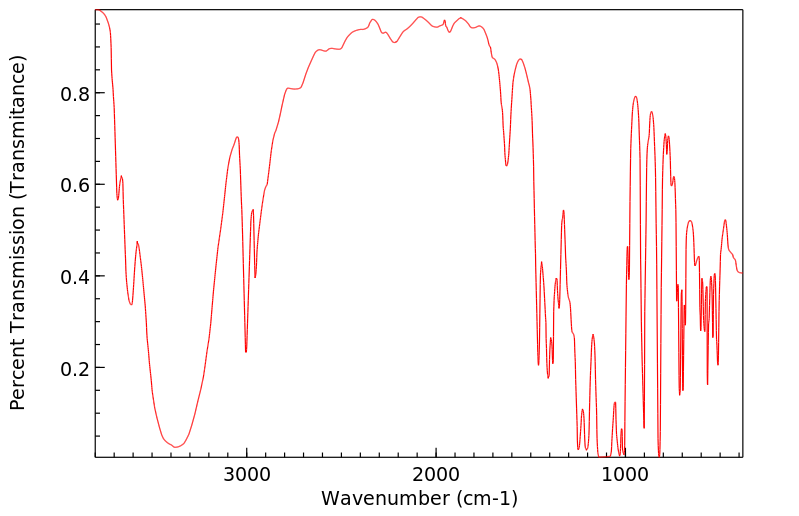
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺-(1S,2S)-1,2-二氢-3-氟邻苯二酚
苏-3-溴-2-丁醇
苏-3-溴-2-丁醇
烯丙基3-氯-2-羟基丙酸酯
溶剂紫36
溴丁醇
水合氯醛
氯醛甜菜碱
氯醛叔丁基半缩醛
氯醛丙基半缩醛
氯二氟乙醛’水合物
氯-(2-氯-3-羟基丙-1-烯基)汞
氘代3-氯-1,2-丙二醇
培氟沙星
四氟乙醇
四氟丙醇
四氟丁二醇
十二氟庚醇
十一氟正己烷-1-醇
六氟异丙醇
六氟丁醇
六氟-1-丙醇
八氟代-1-戊醇
八氟-1,6-己二醇
全氟十醇
全氟-1-辛醇
全氟-1-庚醇
五氟丙醛甲基半缩醛
五氟丙醛水合物
五氟丙醛乙基半缩醛
二溴甘露醇
二氯乙醛水合物
二氯乙氧基合氧钒
二氟乙醛缩半乙醇
乙基3-氟-2-羟基-3-甲基丁酸酯
三溴乙醇
三氟甲基己醇
三氟乙醛缩甲基半醇
三氟乙醛水合物
三氟乙醇
三氟乙基醇-OD
七氟丁醛乙基半缩醛
丁氯醇
rac-2-氯十二烷-1-醇
rac-1-氯十二烷-2-醇
alpha,alpha-二(三氟甲基)-1-氮丙啶甲醇
[2H4]-2-溴-1,3-丙二醇[干冰运输]
[1-氯-3-异丙基氨基-2-丙醇
[1,1-(2)H2]-2-氯乙醇
O-(1,1,3-三氢四氟丙基)-(1-羟基-2,2,2-三氯乙基)甲基膦酸酯