3-丙基环戊烯 | 34067-75-9
中文名称
3-丙基环戊烯
中文别名
——
英文名称
3-propyl-cyclopentene
英文别名
3-Propylcyclopentene
CAS
34067-75-9
化学式
C8H14
mdl
MFCD02666289
分子量
110.199
InChiKey
RBEROAUIKMEYDR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:125.95°C
-
密度:0.7820 (estimate)
-
保留指数:813;816;818;812.6;816.3;840;846;813;816;843
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:8
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902199090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Citterio, Attilio; Minisci, Francesco; Serravalle, Marco, Journal of Chemical Research, Miniprint, 1981, # 7, p. 2174 - 2189摘要:DOI:
-
作为产物:参考文献:名称:Boord et al., Industrial and Engineering Chemistry, 1949, vol. 41, p. 615摘要:DOI:
文献信息
-
Reactions of Carbocations with Unsaturated Hydrocarbons: Electrophilic Alkylation or Hydride Abstraction?作者:Herbert Mayr、Gabriele Lang、Armin R. OfialDOI:10.1021/ja0121538日期:2002.4.1published empirical electrophilicity parameters E of the benzhydrylium ions. Therefore, the linear free energy relationship log k(20 degrees C) = s(E + N) could be employed to characterize the hydride reactivities of the hydrocarbons by the nucleophilicity parameters N and s. The similarity of the slopes s for hydride donors and pi-nucleophiles allows a direct comparison of the reactivities of these different
-
The Preparation of Cyclopentenes and Cyclopentanes. I<sup>1</sup>作者:Grant Crane、Cecil E. Boord、Albert L. HenneDOI:10.1021/ja01224a002日期:1945.8
表征谱图
-
氢谱1HNMR
-
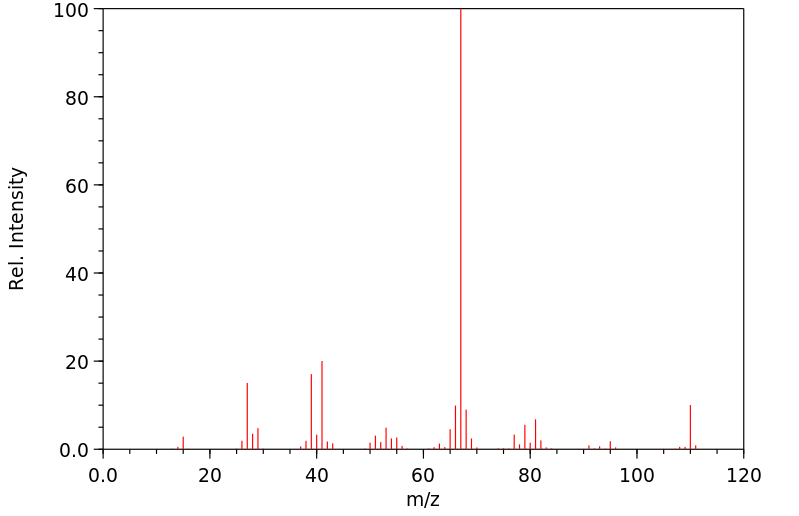
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-