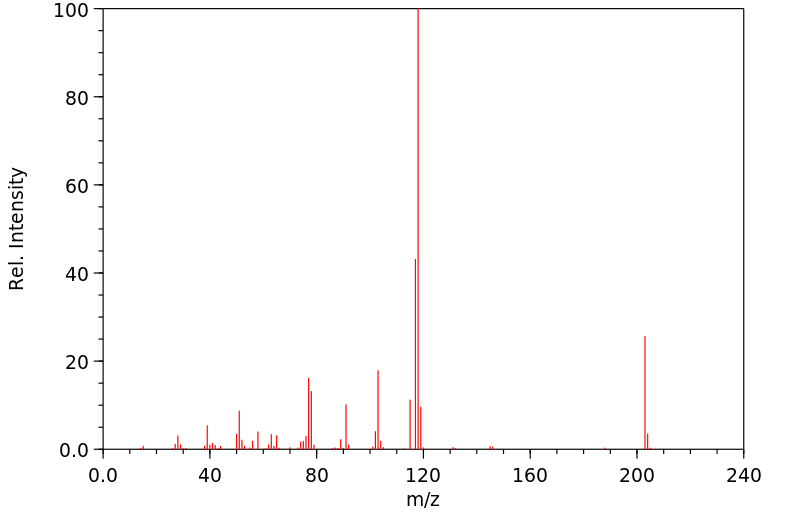
代谢
在服用极高剂量米托苏西米的病人中,有限的研究表明,米托苏西米通过N-去甲基化代谢为N-去甲基米托苏西米(NDM)。米托苏西米过量后出现的深度中枢神经系统抑制被认为是这种代谢物的结果,并且药物的抗惊厥效果可能来自NDM。米托苏西米过量可能呈现双相过程;病人在24小时内苏醒后又陷入昏迷。在一项对长期接受米托苏西米治疗的患者的研究中,NDM的血浆浓度是同期米托苏西米血浆浓度的700倍。基于这项研究,提出了一个暂定的治疗性血浆NDM浓度范围,为10-40微克/毫升;血浆NDM浓度超过40微克/毫升与毒性相关,并且有报道称血浆NDM浓度为150微克/毫升时出现昏迷。
Limited studies in patients who have taken extremely high doses of methsuximide and one study involving a small number of patients receiving methsuximide for the management of epilepsy indicate that the drug is metabolized via N-demethylation to N-demethylmethsuximide (NDM). Profound CNS depression following methsuximide overdosage has been attributed to this metabolite, and it is probable that the anticonvulsant effects of the drug result from NDM. Overdosage of methsuccimide may follow a biphasic course; patients have awakened and relapsed into coma within 24 hours. In one study in patients receiving methsuximide chronically, the plasma concentration of NDM was 700 times greater than the simultaneous plasma concentration of methsuximide. On the basis of this one study, a tentative therapeutic plasma NDM concentration of 10-40 ug/mL has been proposed; plasma NDM concentrations exceeding 40 ug/mL have been associated with toxicity and coma has been reported at plasma NDM concentrations of 150 ug/mL.
来源:Hazardous Substances Data Bank (HSDB)