3-亚甲基十三烷 | 19780-34-8
中文名称
3-亚甲基十三烷
中文别名
2-乙基-1-十二烯
英文名称
3-methylenetridecane
英文别名
2-ethyl-1-dodecene;Tridecane, 3-methylene-;3-methylidenetridecane
CAS
19780-34-8
化学式
C14H28
mdl
——
分子量
196.376
InChiKey
HJXYLVWUVYMJKO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-9.38°C (estimate)
-
沸点:243.29°C (estimate)
-
密度:0.7818 (estimate)
-
保留指数:1407
计算性质
-
辛醇/水分配系数(LogP):7.3
-
重原子数:14
-
可旋转键数:10
-
环数:0.0
-
sp3杂化的碳原子比例:0.86
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-decylbuta-1,3-diene 55235-83-1 C14H26 194.36 1-十二烯十二碳烯α-十二碳烯α-十二碳烯 1-dodecene 112-41-4 C12H24 168.323
反应信息
-
作为产物:描述:参考文献:名称:1,3-二烯与烷基氟化物和格氏试剂的铜催化区域选择性加氢烷基化摘要:由CuCl 2,烷基格氏试剂和1,3-二烯原位生成的铜络合物作为催化活性物质,对通过烷基氟通过CF键裂解的1,3-二烯1,2-加氢烷基化起催化活性作用。烷基被选择性地引入到1,3-二烯的内部碳原子上,从而产生了支链末端烯烃产物。DOI:10.1002/anie.201503288
文献信息
-
Cross-coupling Reaction between Enol Phosphates and Organoaluminium Compounds in the Presence of Palladium(0) Catalyst作者:Kazuhiko Takai、Mitsuyoshi Sato、Koichiro Oshima、Hitosi NozakiDOI:10.1246/bcsj.57.108日期:1984.1The title reaction in the presence of a catalytic amount of tetrakis(triphenylphosphine)palladium(0) affords alkylative coupling products in good to excellent yields in 1,2-dichloroethane at room temperature. This coupling reaction under C(sp2)–O cleavage proceeds stereospecifically. The reaction does not affect a coexisting vinyl sulfide group. This feature enables 1,2- and 1,3-carbonyl transposition
-
Carboncarbon bond formation by cross-coupling of enol phosphates with organoaluminium compounds catalyzed by palladium(O) complex作者:Kazuhiko Takai、Koichiro Oshima、Hitosi NozakiDOI:10.1016/0040-4039(80)80120-1日期:1980.1Trialkylaluminium-mediated alkylation of enol phosphates under the CO bond cleavage is performed stereospecifically in the presence of a catalytic amount of Pd(PPh3)4. Alkenylation and alkynylation are also described.在催化量的Pd(PPh 3)4存在下,立体定向进行三烷基铝介导的烯醇磷酸酯在C = O键断裂下的烷基化反应。还描述了烯基化和炔基化。
-
Elongation and branching of α-olefins by two ethylene molecules作者:Thomas Dietel、Fabian Lukas、Winfried P. Kretschmer、Rhett KempeDOI:10.1126/science.abm5281日期:2022.3.4α-Olefins are important starting materials for the production of plastics, pharmaceuticals, and fine and bulk chemicals. However, the selective synthesis of α-olefins from ethylene, a highly abundant and inexpensive feedstock, is restricted, and thus a broadly applicable selective α-olefin synthesis using ethylene is highly desirable. Here, we report the catalytic reaction of an α-olefin with two ethylene
-
Carbon–Carbon Bond Formation by Cross Coupling of Enol Phosphates or Enol Triflates with Organomanganese Compounds作者:Keigo Fugami、Koichiro Oshima、Kiitiro UtimotoDOI:10.1246/cl.1987.2203日期:1987.11.5Trialkylmanganese-mediated alkylation of enol phosphates is performed in the presence of a catalytic amount of Pd(PPh3)4. The cross coupling reaction catalyzed by Li2MnCl4 between enol triflates and Grignard reagents is also described.
-
New method of ?-alkylation of ?-olefins using dialkylaluminum chlorides with catalytic amounts of Ti, Zr, and Hf complexes作者:U. M. Dzhemilev、A. G. Ibragimov、O. S. Vostrikova、G. A. Tolstikov、L. M. ZelenovaDOI:10.1007/bf00953581日期:1981.2
表征谱图
-
氢谱1HNMR
-
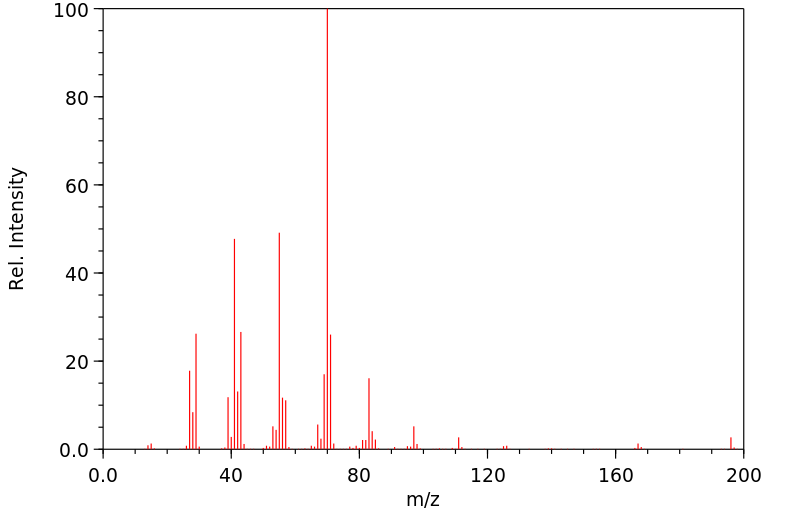
质谱MS
-
碳谱13CNMR
-
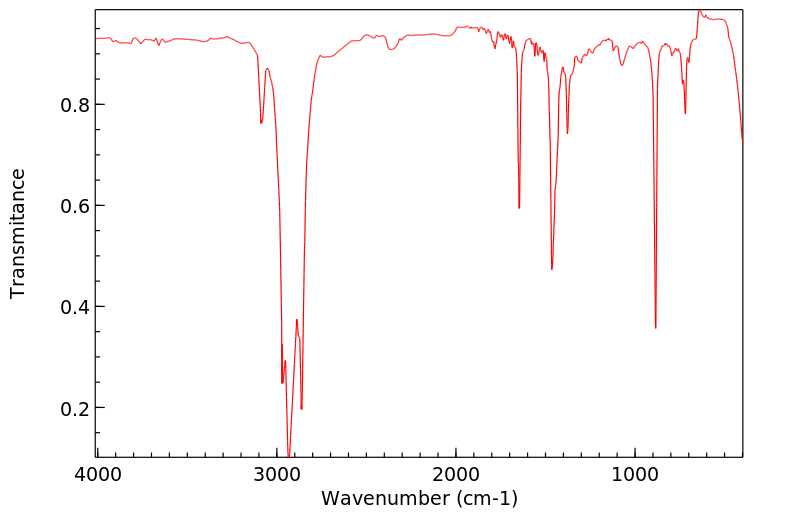
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-