3-氧代-2-戊烷基乙酸酯 | 2983-05-3
中文名称
3-氧代-2-戊烷基乙酸酯
中文别名
1-甲基-2-羰基丁基乙酸酯
英文名称
2-Acetoxy-3-pentanon
英文别名
1-Propionylethyl acetate;3-oxopentan-2-yl acetate
CAS
2983-05-3
化学式
C7H12O3
mdl
MFCD24391517
分子量
144.17
InChiKey
DYGIVYGSRWAUNB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:10
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
SDS
反应信息
-
作为产物:描述:参考文献:名称:Electroorganic chemistry. XXI. Selective formation of .alpha.-acetoxy ketones and general synthesis of 2,3-disubstituted 2-cyclopentenones through the anodic oxidation of enol acetates摘要:DOI:10.1021/ja00854a030
文献信息
-
An Efficient Method for the α-Acetoxylation of Ketones作者:Guosheng Huang、Jinmei Sheng、Xiaolong Li、Mingfang Tang、Botao GaoDOI:10.1055/s-2007-965984日期:2007.4alpha-Acetoxylation of ketones catalyzed by iodobenzene using 30% aqueous hydrogen peroxide and acetic anhydride as the oxidant is an effective and economical method for the preparation of alpha-acetoxy ketones. The reaction gave the products in good yields without the addition of acetic acid and water.
-
Chemistry of dioxiranes. 21. Thermal reactions of dioxiranes作者:Megh Singh、Robert W. MurrayDOI:10.1021/jo00041a036日期:1992.7Thermolysis of dioxiranes in solutions of their parent ketones or in mixtures of the parent ketone and a foreign ketone leads to the formation of esters. The results are explained by postulating a free-radical mechanism involving H atom abstraction from the ketones. The resulting radicals are converted to the observed esters by reaction with acyloxy radicals derived from homolysis of the dioxiranes. Autodecomposition of dimethyldioxirane in acetone solution at room temperature gives methyl acetate at a very slow rate. When catalyzed by BF3 etherate the same decomposition proceeds much more rapidly and is accompanied by acetol formation.
-
A New One-Step Conversion of Ketoximes into α -Acyloxyketones作者:Gaddam Subba Reddy、M. Vivekananda BhattDOI:10.1055/s-1981-29394日期:——
-
Foehlisch, Baldur; Gehrlach, Eberhard; Stezowski, John J., Chemische Berichte, 1986, vol. 119, # 5, p. 1661 - 1682作者:Foehlisch, Baldur、Gehrlach, Eberhard、Stezowski, John J.、Kollat, Petra、Martin, Eveline、Gottstein, WolfgangDOI:——日期:——
-
Competing kc, borderline, ks, and carbonyl addition processes in solvolyses of .alpha.-keto mesylates and triflates. .alpha.-Keto cations. 5作者:Xavier CrearyDOI:10.1021/ja00331a029日期:1984.9
表征谱图
-
氢谱1HNMR
-
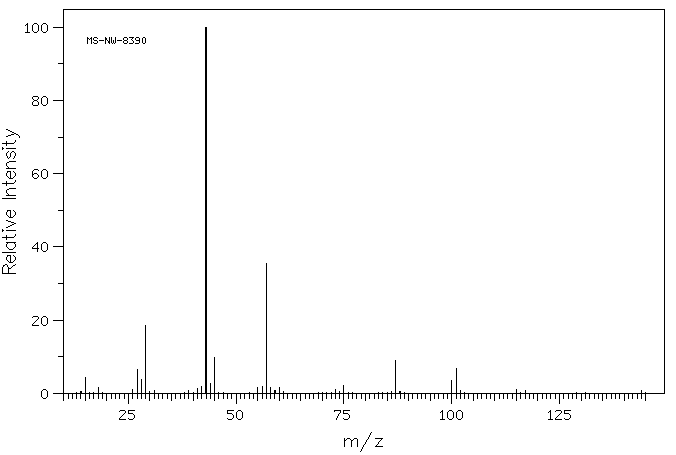
质谱MS
-
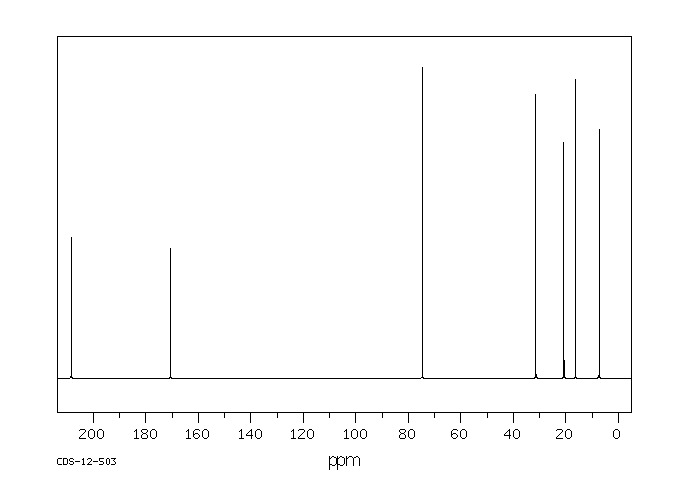
碳谱13CNMR
-
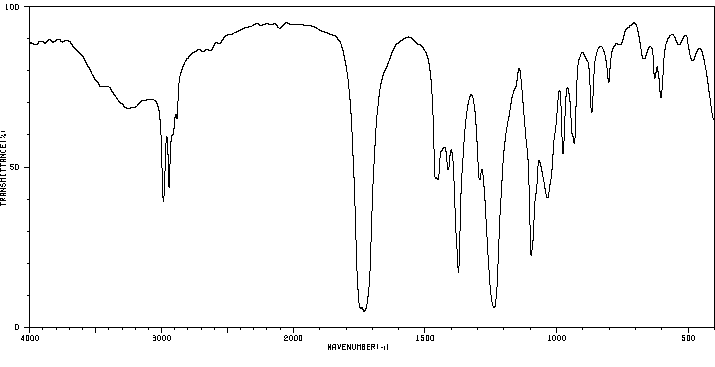
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷