3-氯苯氧乙腈 | 43111-32-6
中文名称
3-氯苯氧乙腈
中文别名
3-氯苯氧基乙腈
英文名称
(3-chloro-phenoxy)-acetonitrile
英文别名
(3-Chlor-phenoxy)-acetonitril;3-Chlorophenoxyacetonitrile;2-(3-chlorophenoxy)acetonitrile
CAS
43111-32-6
化学式
C8H6ClNO
mdl
MFCD00017332
分子量
167.595
InChiKey
NHHNUPMHGVIQQC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:30-34°C
-
沸点:131 °C (1.5 mmHg)
-
密度:1.2295 (rough estimate)
-
闪点:126-128°C/1mm
-
稳定性/保质期:
常规情况下不会分解,也没有任何危险反应。
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:33
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:6.1
-
危险品标志:Xn
-
安全说明:S20,S26,S36/37,S45,S60
-
危险类别码:R20/21/22
-
海关编码:2926909090
-
包装等级:III
-
危险类别:6.1
-
危险品运输编号:3276
SDS
| Name: | 3-Chlorophenoxyacetonitrile Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 43111-32-6 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 43111-32-6 | 3-Chlorophenoxyacetonitrile | 100 | unlisted |
Risk Phrases: 20/21/22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin. May be metabolized to cyanide which in turn acts by inhibiting cytochrome oxidase impairing cellular respiration.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Metabolism may release cyanide, which may result in headache, dizziness, weakness, collapse, unconsciousness and possible death.
May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May be harmful if inhaled. May be metabolized to cyanide which in turns act by inhibiting cytochrome oxidase impairing cellular respiration.
Chronic:
May be metabolized to cyanide which in turn acts by inhibiting cytochrome oxidase impairing cellular respiration. Exposure to small amounts of cyanide compounds over long periods of time is reported to cause loss of appetite, headache, weakness, nausea, dizziness, and symptoms of irritation of the upper respiratory tract and eyes.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Avoid contact with skin and eyes. Keep container tightly closed. Avoid ingestion and inhalation. Use only in a chemical fume hood.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 43111-32-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 126 - 128 deg C @ 1 mmHg
Freezing/Melting Point: 34 - 35 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H6ClNO
Molecular Weight: 167.5011
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation.
Incompatibilities with Other Materials:
Oxidizing agents, strong bases.
Hazardous Decomposition Products:
Hydrogen chloride, nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 43111-32-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-Chlorophenoxyacetonitrile - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
Safety Phrases:
S 36/37 Wear suitable protective clothing and
gloves.
WGK (Water Danger/Protection)
CAS# 43111-32-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 43111-32-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 43111-32-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-氯苯酚 3-Chlorophenol 108-43-0 C6H5ClO 128.558 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-(3-chlorophenoxy)propionitrile 6560-15-2 C9H8ClNO 181.622 2-(3-氯苯氧基)硫代乙酰胺 2-(3-chlorophenoxy) ethanethioamide 35370-95-7 C8H8ClNOS 201.677
反应信息
-
作为反应物:描述:3-氯苯氧乙腈 在 哌啶 、 sodium ethanolate 作用下, 以 乙醇 为溶剂, 反应 7.0h, 生成 Isonicotinic acid [2-(3-chloro-phenoxy)-1-{[1-pyridin-3-yl-meth-(E)-ylidene]-amino}-eth-(E)-ylidene]-hydrazide参考文献:名称:Synthesis and antimycobacterial activity of some isonicotinoylhydrazones摘要:A series of isonicotinoylhydrazones 2 were prepared by addition of some aryloxyacetonitriles with isonicotinoylhydrazine in basic medium. These compounds have been further reacted with pyridinecarboxaldehydes to give the corresponding pyridylmethyleneamino derivatives 3-5. The new synthesized hydrazones and their pyridylmethyleneamino derivatives were tested for their activity against mycobacteria, Gram-positive and Gram-negative bacteria. The cytotoxicity was also tested. Several compounds showed a good activity against Mycobacterium tuberculosis H37Rv and some isonycotinoylhydrazones 2 showed a moderate activity against a clinically isolated M. tuberculosis which was isoniazid resistant. (C) 1999 Editions scientifiques et medicales Elsevier SAS.DOI:10.1016/s0223-5234(99)00124-5
-
作为产物:描述:参考文献:名称:通过 Pd/降冰片烯催化,天然氨基定向伯胺间位选择性 C–H 芳基化摘要:游离伯胺中远程 C-H 键的选择性官能化为药物的后期多样化带来了重大前景。然而,迄今为止,缺乏额外导向基团的胺底物间位的直接官能化仍未得到充分探索。在这封信中,我们展示了使用芳基碘化物对游离伯胺衍生物进行成功的间位-C-H芳基化,从而获得了具有合成价值的产率。这种元选择性 C-H 功能化是通过涉及天然氨基定向 Pd 催化的七元环金属化的序列实现的,然后利用降冰片烯型瞬时介体。DOI:10.1021/acs.orglett.4c00721
文献信息
-
Combinatorial Synthesis and in Vitro Evaluation of a Biaryl Hydroxyketone Library as Antivirulence Agents against MRSA作者:Guanping Yu、David Kuo、Menachem Shoham、Rajesh ViswanathanDOI:10.1021/co400142t日期:2014.2.10crystallized. This strategy affords a range of biarylhydroxyketones in a single step. This is the first collective synthetic study documenting access to this class of compounds through a single synthetic operation. In vitro efficacy of compounds in this library was evaluated by a rabbit erythrocyte hemolysis assay. The most efficacious compound, 4f-12, inhibits hemolysis by 98.1 +/- 0.1% compared to抗生素抗性加上新抗生素的发展减少,因此有必要寻找新型抗菌剂。抗毒剂是常规抗生素的替代品。在这项工作中,我们报告了针对最广泛的细菌病原体耐甲氧西林金黄色葡萄球菌(MRSA)的小分子抗毒剂家族。结构-活性关系的研究导致了148位联芳基羟基酮库的简明合成方法的发展。酰化键形成过程提供间苯二酚(1)和芳氧基乙腈(2)作为合成子。进行路易斯酸活化的Friedel-Crafts酰化步骤,其中ZnCl2的腈官能度为2,然后亲核力为1,从而以优异的收率获得了联芳基羟基酮。大量产品结晶。此策略可在一个步骤中提供一系列联芳基羟基酮。这是第一个集体合成研究,它记录了通过单一合成操作对此类化合物的访问。该化合物在该文库中的体外功效通过兔红细胞溶血试验评估。与不存在该化合物的对照相比,最有效的化合物4f-12抑制溶血98.1 +/- 0.1%。
-
A new synthesis of 2-aryloxypropionic acids derivatives via selective mono-c-methylation of methyl aryloxyacetates and aryloxyacetonitriles with dimethyl carbonate作者:Andrea Bomben、Carlos A. Marques、Maurizio Selva、Pietro TundoDOI:10.1016/0040-4020(95)00718-n日期:1995.10A one-pot procedure for the mono-C-methylation of methyl aryloxyacetates and aryloxyaceto nitnles by dimethyl carbonate (DMC) is reported The reaction is earned out in an autoclave at high temperatures (180–200 ° C) and in the presence of a base (K2CO3 or bart-BuOK). Although DMC is used either as the alkylating agent or as the solvent (30 molar excess with respect to the substrates). The selectivity
-
POLY(ADP-RIBOSE) POLYMERASE INHIBITORS CONSISTING OF PYRIMIDINE DERIVATIVES申请人:Meiji Seika Kaisha, Ltd.公开号:EP1142881A1公开(公告)日:2001-10-10A medicament for therapeutic and/or preventive treatment of a brain disease, which comprises a compound represented by the following general formula (I) or a pharmaceutically acceptable salt thereof as an active ingredient: wherein R represents hydrogen atom, a C1-C8 alkyl group, a substituted C1-C8 alkyl group, an aryl group, a substituted aryl group, an aryl(C1-C8)alkyl group and the like; Y represents hydrogen atom or -C(R2)R3 (R2 and R3 represent hydrogen atom, a C1-C8 alkyl group, a C1-C8 alkoxy(C1-C8)alkyl group, a hydroxy(C1-C8)alkyl group and the like); symbol "a" represents single bond when Y represents hydrogen atom, or "a" represents double bound when Y represents -C(R2)R3; -A-B- represents -CH2-CH2-, -S-CH2-, -O-CH2-, -CH2-S-, -CH2-O-, -SO-CH2-, -CH2-SO-, -SO2-CH2-, or -CH2-SO2-; and Z represents -CH2- or single bond.一种用于治疗和/或预防脑部疾病的药物,其活性成分包括下式通式(I)所代表的化合物或其药学上可接受的盐: 其中 R 代表氢原子、C1-C8 烷基、取代的 C1-C8 烷基、芳基、取代的芳基、芳基(C1-C8)烷基等;Y 代表氢原子或 -C(R2)R3 (R2 和 R3 代表氢原子、C1-C8 烷基、C1-C8 烷氧基(C1-C8)烷基、羟基(C1-C8)烷基等);符号 "a "在 Y 代表氢原子时代表单键,或 "a "在 Y 代表-C(R2)R3 时代表双键;-A-B-代表-CH2- -、-S- -、-O- -、- -S-、- -O-、-SO- -、- -SO-、-SO2-或- -SO2-;Z 代表- -或单键。
-
Julia, Bulletin de la Societe Chimique de France, 1956, p. 1365作者:JuliaDOI:——日期:——
-
A New and Efficient Synthesis of Phthalazin-1(2H)-ones作者:A. M. Bernard、M. T. Cocco、C. Congiu、V. Onnis、P. P. PirasDOI:10.1055/s-1998-2036日期:1998.3
表征谱图
-
氢谱1HNMR
-
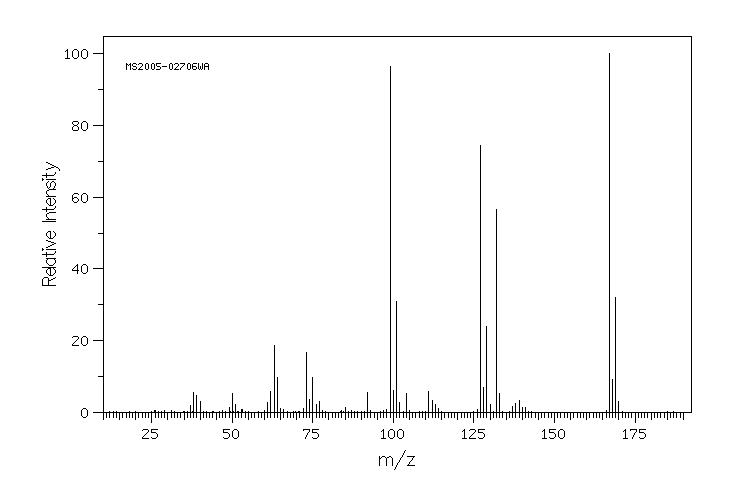
质谱MS
-
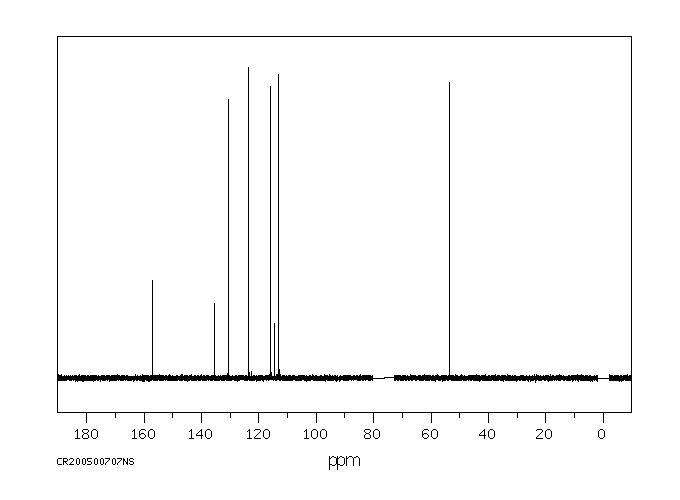
碳谱13CNMR
-
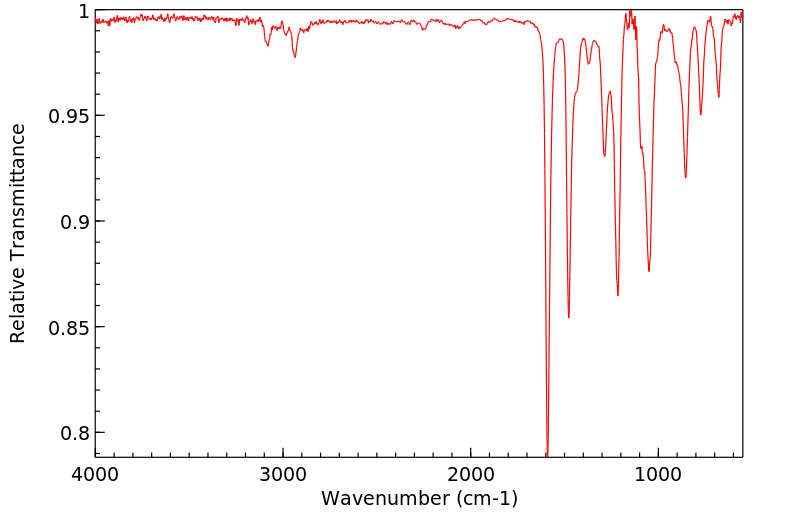
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯