3-甲基-2,5-恶唑烷二酮 | 5840-76-6
中文名称
3-甲基-2,5-恶唑烷二酮
中文别名
——
英文名称
sarcosine-N-carboxyanhydride
英文别名
sarcosine NCA;Sar NCA;3-methyl-2,5-oxazolidinedione;3-Methyl-2,5-oxazolidine-dione;3-methyl-1,3-oxazolidine-2,5-dione
CAS
5840-76-6
化学式
C4H5NO3
mdl
MFCD02684172
分子量
115.089
InChiKey
PMPAXURTFMSZSN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:99-100 °C
-
沸点:138.7±23.0 °C(Predicted)
-
密度:1.373±0.06 g/cm3(Predicted)
-
溶解度:二甲基亚砜(微溶)
计算性质
-
辛醇/水分配系数(LogP):-0.3
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:46.6
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险性防范说明:P261,P280,P305+P351+P338,P304+P340,P405,P501
-
危险性描述:H315,H319,H335
-
储存条件:保存条件:2-8°C,惰性气体保护。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— N-(methoxycarbonyl)-N-methylglycine 116714-27-3 C5H9NO4 147.131
反应信息
-
作为反应物:描述:参考文献:名称:用聚合物催化剂聚合氨基酸衍生物。三、聚(N-乙基甘氨酸)二乙酰胺诱导的链效应聚合摘要:研究了由具有末端仲氨基的预制聚(N-乙基甘氨酸)二乙酰胺诱导的 D,L-β-苯丙氨酸 NCA 的聚合。聚合反应比由类似碱强度的低分子量胺引发的相同反应快得多。因此,链效应对聚(N-乙基甘氨酸)二乙酰胺以及聚肌氨酸二乙酰胺起作用,Bamford 等人。发现了连锁效应机制。聚合速率取决于聚合物催化剂的聚合度。速率提高归因于 NCA 通过形成氢键吸附到聚合物链的酰胺羰基上。在从聚肌氨酸二乙酰胺到聚(N-乙基甘氨酸)二乙酰胺的过程中,NCA 在聚合物链上的吸收率几乎没有受到影响。然而,与聚肌氨酸二乙酰胺相比,使用聚(N-乙基甘氨酸)二乙酰胺吸附的 NCA 与末端碱基之间的反应更困难。这可能与聚合物链柔韧性的差异有关。DOI:10.1002/bip.1969.360070609
-
作为产物:参考文献:名称:N-取代的N-羧酐的N-杂环卡宾介导的开环聚合的环状聚(α-拟肽)及其嵌段共聚物摘要:N-取代的 N-羧基酐 ((N)R-NCA) 的 N-杂环卡宾 (NHC) 介导的开环聚合 (ROP) 产生具有受控分子量 (M(n) = 3-30 kg mol(-1)) 和窄分子量分布 (PDI = 1.04-1.12)。该反应表现出具有最小链转移的活性聚合特征。这使得可以通过顺序单体添加轻松合成环状二嵌段共聚(α-拟肽)。通过 MALDI-TOF 质谱和特性粘度测量验证了环状聚合物结构。Mark-Houwink-Sakurada 绘图分析显示,由 NHC 介导的聚合制备的环状聚 (α-拟肽) 比由伯胺引发的聚合制备的线性类似物具有更低的特性粘度。DOI:10.1021/ja907380d
-
作为试剂:描述:3-[[5-(Aminomethyl)tetrahydro-3-furanyl]thio]-6-(1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid 在 6-(1-Hydroxyethyl)-4-methyl-7-oxo-3-[[tetrahydro-5-[[[(methylamino)acetyl]amino]methyl]-3-furanyl]thio]-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid 作用下, 以 Sodium phosphate, tribasic- 、 3-甲基-2,5-恶唑烷二酮 、 1,4-二氧六环 为溶剂, 生成 6-(1-Hydroxyethyl)-4-methyl-7-oxo-3-[[tetrahydro-5-[[[(methylamino)acetyl]amino]methyl]-3-furanyl]thio]-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid参考文献:名称:2-thiosubstituted carbapenems摘要:通式为:##STR1##的碳青霉烯类抗生素化合物,其中基团##STR2##是4、5或6元单、双或三取代的含氧或硫的环;其中Z是氧、硫、亚砜和砜,其药物组成物可用于治疗细菌感染,制备该化合物的过程以及在该过程中有用的新中间体。公开号:US05750735A1
文献信息
-
Über die Reaktion von α‐Aminosäure‐ <i>N</i> ‐carbonsäure‐anhydriden mit <i>N</i> ‐silylierten prim. und sek. Aminen作者:Hans R. Kricheldorf、Gerd GreberDOI:10.1002/cber.19711041022日期:1971.10Die Umsetzung von Aminosäure-N-carbonsäure-anhydriden (1) (Oxazolidindionen-(2.5)) mit N-silylierten Aminen führt nicht wie die Reaktion mit Aminen zu Oligo- oder Polypeptiden. Als Reaktionsprodukte entstehen N-Trimethylsiloxycarbonyl-aminosäure-amide (2) neben Hydantoinsäure-silylestern (12), die nach Hydrolyse als Aminosäureamide (4) und Hydantoinsäuren (5) isoliert wurden. Die Umsetzung N-silylierter
-
Alkali-metal hexamethyldisilazide initiated polymerization on alpha-amino acid N-substituted N-carboxyanhydrides for facile polypeptoid synthesis作者:Yueming Wu、Min Zhou、Kang Chen、Sheng Chen、Ximian Xiao、Zhemin Ji、Jingcheng Zou、Runhui LiuDOI:10.1016/j.cclet.2021.02.039日期:2021.5been explored as mimics of polypeptides, owing to polypeptoids’ superior stability upon proteolysis. Polypeptoids can be synthesized from one-pot ring-opening polymerization of amino acid N-substituted N-carboxyanhydrides (NNCAs). However, the speed of polymerization of NNCAs can be very slow, especially for NNCAs bearing a bulky N-substitution group. This hindered the exploration on polypeptoids with
-
Secondary-Structure-Driven Self-Assembly of Reactive Polypept(o)ides: Controlling Size, Shape, and Function of Core Cross-Linked Nanostructures作者:Kristina Klinker、Olga Schäfer、David Huesmann、Tobias Bauer、Leon Capelôa、Lydia Braun、Natascha Stergiou、Meike Schinnerer、Anjaneyulu Dirisala、Kanjiro Miyata、Kensuke Osada、Horacio Cabral、Kazunori Kataoka、Matthias BarzDOI:10.1002/anie.201702624日期:2017.8.1function of polymeric nanostructures during self‐assembly remains a challenge in materials as well as biomedical science, especially when independent control over particle properties is desired. Herein, we report on nanostructures derived from amphiphilic block copolypept(o)ides by secondary‐structure‐directed self‐assembly, presenting a strategy to adjust core polarity and function separately from particle
-
Directed Interactions of Block Copolypept(o)ides with Mannose-binding Receptors: PeptoMicelles Targeted to Cells of the Innate Immune System作者:Philipp Heller、Nicole Mohr、Alexander Birke、Benjamin Weber、Angelika Reske-Kunz、Matthias Bros、Matthias BarzDOI:10.1002/mabi.201400417日期:2015.1acid N‐carboxyanhydrides. These amphiphilic block copolypept(o)ides self‐assemble into multivalent PeptoMicelles and bind to mannose‐binding receptors as expressed by dendritic cells. Mannosylated micelles showed enhanced cell uptake in DC 2.4 cells and in bone marrow‐derived dendritic cells (BMDCs) and therefore appear to be a suitable platform for immune modulation.
-
Multidentate Polysarcosine-Based Ligands for Water-Soluble Quantum Dots作者:Ana Fokina、Kristina Klinker、Lydia Braun、Byeong Guk Jeong、Wan Ki Bae、Matthias Barz、Rudolf ZentelDOI:10.1021/acs.macromol.6b00582日期:2016.5.24We describe the synthesis of heterotelechelic polysarcosine polymers and their use as multidentate ligands in the preparation of stable water-soluble quantum dots (QDs). Orthogonally functionalized polysarcosine with amine and dibenzocyclooctyl (DBCO) end groups is obtained by ring-opening polymerization of N-methylglycine N-carboxyanhydride with DBCO amine as initiator. In a first postpolymerization我们描述了杂telechelic聚肌氨酸聚合物的合成及其在制备稳定的水溶性量子点(QDs)中作为多齿配体的用途。通过N-甲基甘氨酸N的开环聚合获得具有胺和二苯并环辛基(DBCO)端基的正交官能化聚肌氨酸-用DBCO胺作为引发剂的羧基酐。在第一个后聚合修饰步骤中,通过胺末端的修饰来调节聚合物配体的未来生物活性。然后,在第二个聚合后修饰步骤中,通过应变促进的叠氮化物-炔烃环加成反应(SPAAC)将叠氮化物官能化的二齿和三齿锚固化合物引入聚肌氨酸的DBCO末端。通过锚固化合物的单独合成,可以确保将确定数目的多个锚固基团可重复地引入所有研究的聚合物中。最后,将获得的多齿聚合物配体成功用于配体交换程序中,以产生稳定的水溶性QD。由于基于聚肌氨酸的配体可以提供生物相容性,防止非特异性相互作用,离体分析或生物成像。
表征谱图
-
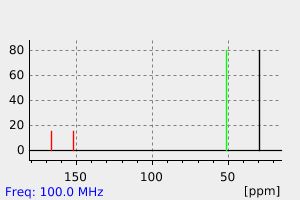
氢谱1HNMR
-
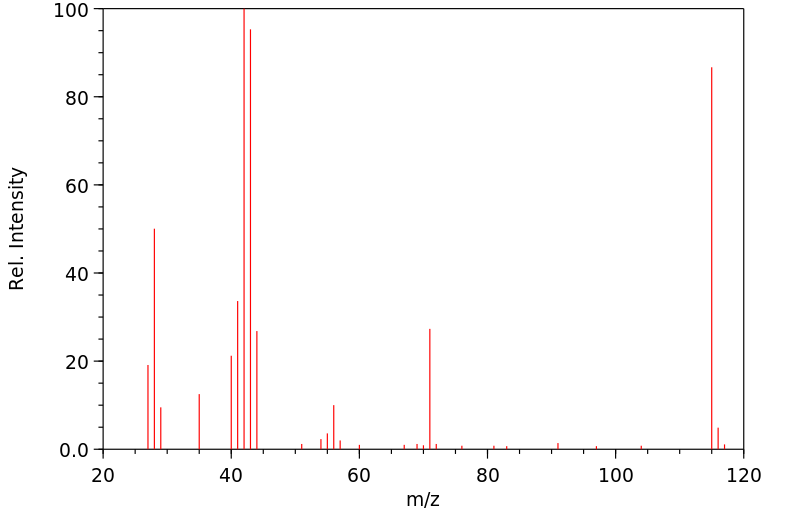
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸