盐酸尼卡地平 | 69441-18-5
中文名称
盐酸尼卡地平
中文别名
盐酸诺拉替坦;2,6-二甲基-4-(3-硝基苯基)-1,4-二氢-3,5-吡啶二羧酸2-[甲基(苄基)氨基]乙基甲基酯盐酸盐
英文名称
nicardipine hydrochloride
英文别名
Loxen;5-O-[2-[benzyl(methyl)amino]ethyl] 3-O-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate;hydron;chloride
CAS
69441-18-5;54527-84-3
化学式
C26H29N3O6*ClH
mdl
MFCD00057327
分子量
515.994
InChiKey
AIKVCUNQWYTVTO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:176-1780C
-
溶解度:二甲基亚砜:~1 mg/mL
-
碰撞截面:211.9 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
计算性质
-
辛醇/水分配系数(LogP):3.56
-
重原子数:36
-
可旋转键数:10
-
环数:3.0
-
sp3杂化的碳原子比例:0.307
-
拓扑面积:114
-
氢给体数:2
-
氢受体数:8
安全信息
-
危险等级:6.1(b)
制备方法与用途
性质
本品为淡黄色粉末或黄色结晶性粉末;无臭,几乎无味。
用途主要用于治疗:
- 高血压,单独应用或与其他药物合并应用;
- 心绞痛,单独应用或与其他药物合并应用。
主要成分为盐酸尼卡地平,其化学名称为:2,6-二甲基-4-(3-硝基苯基)-1,4-二氢吡啶-3,5-二羧酸,3-[β-(N-苄基-N-甲氨基)]乙酯-5-甲酯盐酸盐。分子式:C₂₆H₂₉N₃O₆·HCl 分子量:515.99
药理作用: 本品为钙拮抗剂、血管扩张药,抑制心肌与血管平滑肌的跨膜钙离子内流而不改变血钙浓度。对血管的选择性较强,在动物实验中显示可扩张冠状血管平滑肌,并且在产生此作用时未见对心肌产生负性肌力作用。人体试验表明本品能够降低周围血管阻力,尤其是在高血压患者大于正常血压者中降压时伴有反射性心率加快。此外,本品还可使心脏射血分数及心排血量增多而左室舒张末压改变不多,并能增加冠状血流。
药物相互作用- 与β阻滞剂同用耐受良好;
- 与西咪替丁同用可使本品血药浓度增高;
- 与地高辛合用未见地高辛血药浓度增高,但需测定地高辛血药浓度;
- 与环孢素合用时环孢素血药浓度增高;
- 在体外治疗浓度的呋塞米、普萘洛尔、双嘧达莫、华法林、奎尼丁、萘普生加于人体血浆中不改变本品的蛋白结合率。
- 较常见者有脚肿、头晕、头痛、脸红。均为血管扩张的结果。
- 较少见者有心悸、心动过速、心绞痛加重,通常是反射性心动过速所致;减小剂量或加用β-阻滞药可以纠正。
- 少见者有恶心、口干、便秘、乏力、皮疹等。
禁忌症: 对本品有过敏反应者;重度主动脉瓣狭窄、颅内出血尚未完全止血者、脑中风等颅压患者。
储存置棕色玻璃瓶中,密闭保存。
参考资料[血管扩张药 用途](http://baike.baidu.com/link?url=qjrKdFbpHxYy5cCgN5tJqBw1YCMe9OxSewk_Ya2J5oqBsZhlJ-CFPvj7THUZFlFns94yzZJvERNDQip-iPpOeCDIlsOsOXADqO3_4bVGnxn5iES5ilCmoZgRTFsZs0mD31p-QYRnBjZdmPvBOm1Fq_)
反应信息
-
作为反应物:参考文献:名称:Formation of DNA-damaging N-nitroso compounds from the interaction of calcium-channel blockers with nitrite摘要:A large number of drugs have been shown to react with nitrite to give genotoxic-carcinogenic N-nitroso compounds (NOC). However, the majority of drugs remain to be examined in this respect, among which calcium-channel blockers, all theoretically nitrosatable and widely used in the therapy of hypertension and other cardiovascular diseases. In this preliminary investigation, seven calcium-channel blockers have been examined either for their in vitro nitrosation according to the procedure recommended by the WHO, or for occurrence of liver DNA fragmentation, as detected by the Comet assay, in rats given by gavage 1/2 LD50 of the drug and 80 mg/kg of sodium nitrite. After 6 h incubation the yields of NOC formed in vitro from nicardipine, nifedipine, nimodipine and nitrendipine ranged from 37 to 45% of the theoretical one, whereas the yields of NOC formed from diltiazem, gallopamil and verapamil ranged from 2 to 5%. In vivo, as compared with the effect of the same dose of the drug alone, a significant increase of both tail length and tail moment, indicative of an increased frequency of DNA single-strand breaks and alkali-labile sites, was produced in rat liver DNA by the administration with nitrite of gallopamil, nifedipine, nimodipine and nitrendipine, the ratio [tail length of drug + NaNO2/tail length of drug alone] being 3.2 for nimodipine, 3.1 for gallopamil 2.2 for nifedipine, and 2.1 for nitrendipine. Even if present, the increase in the degree of DNA fragmentation did not reach the statistical significance in rats given with nitrite nicardipine, diltiazem and verapamil. Further studies should be performed to investigate the formation of NOC in conditions Simulating those occurring in the stomach of humans treated with a therapeutic dose, and to quantitate their genotoxic potency. (c) 2007 Elsevier Ireland Ltd. All rights reserved.DOI:10.1016/j.tox.2007.06.001
-
作为产物:参考文献:名称:Synthesis of asymmetric 4-aryl-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylates with vasodilating and antihypertensive activities.摘要:合成了非对称的4-芳基-1,4-二氢-2,6-二甲基-3,5-吡啶二羧酸酯,其中含有2-乙烯二氧丙基、2-氧丙基或环丙基甲基作为酯基,以及相关衍生物,并进行了在麻醉开放胸腔犬中的扩血管活性及在意识清醒的自发性高血压大鼠中的抗高血压活性测试。环丙基甲基甲基1,4-二氢-2,6-二甲基-4-(3-硝基苯基)-3,5-吡啶二羧酸酯(5f,MPC-2101)和甲基2-氧丙基1,4-二氢-2,6-二甲基-4-(2-硝基苯基)-3,5-吡啶二羧酸酯(8i,MPC-1304)分别表现出强效的脑扩血管和抗高血压活性。在3.0 μg/kg的静脉给药后,5f对脊椎血流的最大增加为221.4%,而硝苯地平和尼卡地平氯化物分别为187.0%和166.3%。8i在0.3和1.0 mg/kg口服给药后的收缩压最大下降分别为42.2和54.0 mmHg,而硝苯地平、尼卡地平和肼苯噻啶氯化物在3.0 mg/kg时的最大下降分别为23.3、16.8和24.5 mmHg。5f和8i的显著扩血管和抗高血压效果持续时间比硝苯地平和尼卡地平更长。DOI:10.1248/cpb.34.1589
文献信息
-
[EN] HETEROCYCLIC COMPOUNDS FOR THE TREATMENT OF STRESS-RELATED CONDITIONS<br/>[FR] COMPOSÉS HÉTÉROCYCLIQUES POUR LE TRAITEMENT D'ÉTATS LIÉS AU STRESS申请人:OTSUKA PHARMA CO LTD公开号:WO2010137738A1公开(公告)日:2010-12-02The present invention provides a novel heterocyclic compound. A heterocyclic compound represented by general formula (1) wherein, R1 and R2, each independently represent hydrogen; a phenyl lower alkyl group that may have a substituent(s) selected from the group consisting of a lower alkyl group and the like on a benzene ring and/or a lower alkyl group; or a cyclo C3-C8 alkyl lower alkyl group; or the like; R3 represents a lower alkynyl group or the like; R4 represents a phenyl group that may have a substituent(s) selected from the group consisting of a 1,3,4-oxadiazolyl group that may have e.g., halogen or a heterocyclic group selected from pyridyl group and the like; the heterocyclic group may have at least one substituent(s) selected from a lower alkoxy group and the like or a salt thereof.
-
Dibenzylamine compound and medicinal use thereof申请人:Maeda Kimiya公开号:US20050059810A1公开(公告)日:2005-03-17A dibenzylamine compound represented by the formula (1) wherein R 1 and R 2 are each a C 1-6 alkyl group optionally substituted by halogen atoms and the like; R 3 , R 4 and R 5 are each a hydrogen atom, a halogen atom and the like, or R 3 and R 4 may form, together with carbon atoms bonded thereto, a homocyclic or heterocyclic ring optionally having substituent(s); A is —N(R 7 ) (R 8 ) and the like; ring B is an aryl group or a heterocyclic residue; R 6 is a hydrogen atom, a halogen atom, a nitro group, a C 1-6 alkyl group and the like; n is an integer of 1 to 3, a prodrug thereof and a pharmaceutically acceptable salt thereof show selective and potent CETP inhibitory activity, and therefore, they can be provided as therapeutic or prophylactic agents for hyperlipidemia or arteriosclerosis and the like.
-
Piperidine derivatives申请人:——公开号:US20040142956A1公开(公告)日:2004-07-22Compounds, compositions and methods are provided that are useful in the treatment or prevention of conditions or disorders associated with a neuropeptide receptor. The subject methods are particularly useful in the treatment and/or prevention of endocrine, metabolic, cardiovascular, neurologic, psychiatric, gastrointestinal, genitourinary and other disorders.提供了在治疗或预防与神经肽受体相关的疾病或疾病的化合物、组合物和方法。这些方法特别适用于治疗和/或预防内分泌、代谢、心血管、神经、精神、消化、泌尿生殖和其他疾病。
-
Nitrogen-containing fused ring compounds and use thereof申请人:Hirata Kazuyuki公开号:US20070010670A1公开(公告)日:2007-01-11A URAT1 activity inhibitor containing a nitrogen-containing fused ring compound represented by the following formula [1]: wherein each symbol is as defined in the description. The present invention is useful for the prophylaxis or treatment of pathology showing involvement of uric acid, such as hyperuricemia, gouty tophus, acute gouty arthritis, chronic gouty arthritis, gouty kidney, urolithiasis, renal function disorder, coronary artery disease, ischemic heart disease and the like.
-
Ester derivatives and medicinal use thereof申请人:Hagiwara Atsushi公开号:US20060089392A1公开(公告)日:2006-04-27The present invention relates to an ester represented by the formula [1]: or its pharmaceutically acceptable salt, or use of the same. The compound represented by the formula [1] or its pharmaceutically acceptable salt is useful as an agent for the treatment or prophylaxis of hyperlipidemia or the like, since it disappears very rapidly in the living body and has an excellent MTP inhibitory activity.
表征谱图
-
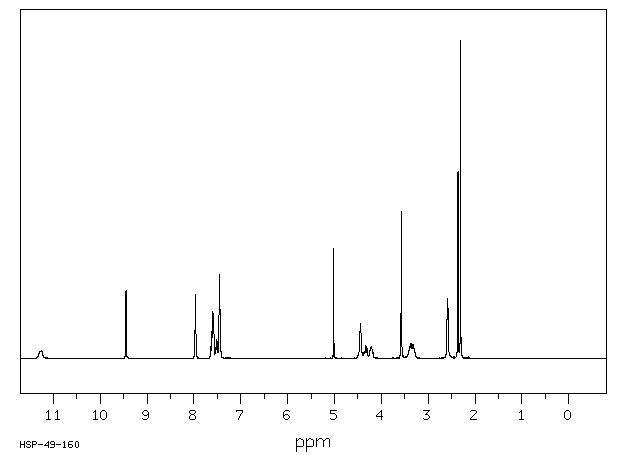
氢谱1HNMR
-
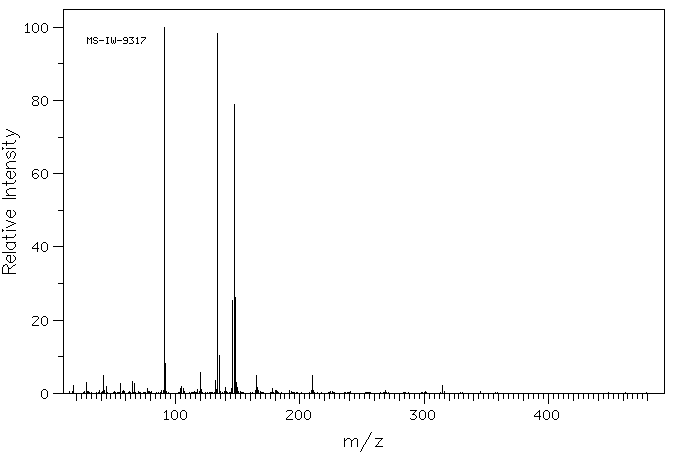
质谱MS
-
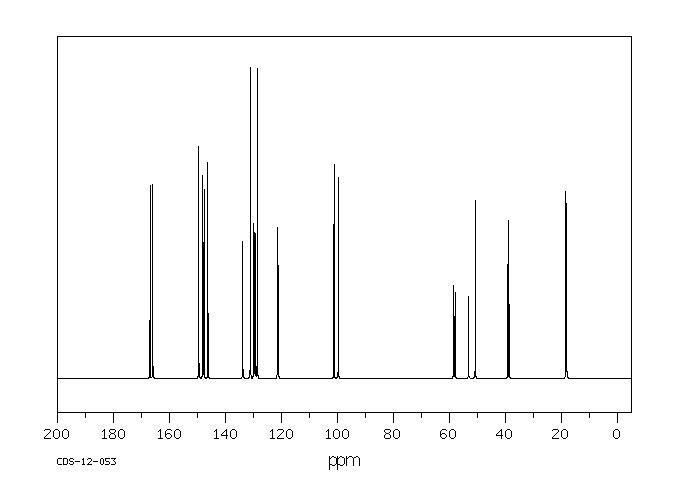
碳谱13CNMR
-
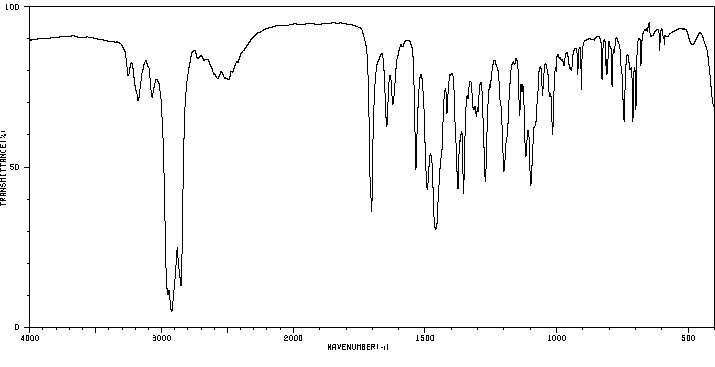
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-