3-羟基-2-吡喃酮 | 496-64-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:93.8°C
-
沸点:149.97°C (rough estimate)
-
密度:1.2552 (rough estimate)
-
溶解度:可溶于氯仿、甲醇(少许)
-
LogP:-0.763 (est)
-
物理描述:Solid
-
保留指数:959;959;959
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2932999099
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:室温和干燥环境
SDS
Section 1. Identification of the substance
Product Name: 3-Hydroxy-2-pyrone
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 3-Hydroxy-2-pyrone
CAS number: 496-64-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C5H4O3
Molecular weight: 112.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2H-吡喃-2-酮 pyran-2-one 504-31-4 C5H4O2 96.0856 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-甲氧基-2H-吡喃-2-酮 3-Methoxy-2H-pyran-2-on 51270-28-1 C6H6O3 126.112 —— 4-chloro-3-hydroxy-2-pyrone 159395-50-3 C5H3ClO3 146.53 —— 4-bromo-3-hydroxy-2-pyrone 98136-26-6 C5H3BrO3 190.981 —— 3-hydroxy-4-methyl-2H-pyran-2-one 942499-01-6 C6H6O3 126.112 —— 3-Trimethylsilanyloxy-pyran-2-one 108298-46-0 C8H12O3Si 184.267
反应信息
-
作为反应物:描述:参考文献:名称:3-羟基-2-吡喃酮与手性丙烯酸酯衍生物的不对称碱催化Diels-Alder反应摘要:在金鸡纳生物碱作为催化剂的存在下,3-羟基-2-吡喃酮与手性N-丙烯酰基恶唑烷酮的狄尔斯-阿尔德反应以几乎定量的产率提供了具有高非对映选择性(高达95%de)的双环内酯加合物。DOI:10.1016/s0040-4039(97)10843-7
-
作为产物:描述:维生素 C 在 sodium nitrite 作用下, 反应 0.5h, 生成 3-羟基-2-吡喃酮参考文献:名称:Obata, Hitoshi; Tanigaki, Hiroshi; Tanishita, Jun-ichi, Agricultural and Biological Chemistry, 1983, vol. 47, # 2, p. 419 - 420摘要:DOI:
文献信息
-
Unified Total Syntheses of Rhamnofolane, Tigliane, and Daphnane Diterpenoids作者:Akira Hirose、Ayumu Watanabe、Kohei Ogino、Masanori Nagatomo、Masayuki InoueDOI:10.1021/jacs.1c06450日期:2021.8.11ABC-ring 6 by detaching the three-carbon units and the oxygen-appended groups. Intermediate 6 with six stereocenters was assembled from four achiral fragments in 12 steps by integrating three powerful transformations, as follows: (i) asymmetric Diels–Alder reaction to induce formation of the C-ring; (ii) π-allyl Stille coupling reaction to set the trisubstituted E-olefin of the B-ring; and (iii) Eu(fod)3-promotedRhamnofolane、tigliane 和 daphnane diterpenoids 是结构复杂的天然产物,具有多种氧官能团,使它们在合成上具有挑战性。虽然这些二萜类化合物共享一个5/7/6反式稠合环系统(ABC 环),但 C 环上 C13 和 C14 位的三碳取代以及附加的氧官能团在它们之间是不同的,占这些天然产物的不同生物活性。在这里,我们开发了一种新的、统一的策略,用于快速全合成这三个家族的五个代表性成员,crotophorbolone ( 1 )、langduin A ( 2 )、prostratin ( 3 )、resiniferatoxin ( 4 ) 和 tinyatoxin ( 5))。逆合成,1 - 5分别简化为它们共同的ABC-环6通过卸下三碳单元和氧附属基团。具有六个立体中心的中间体6由四个非手性片段分 12 步通过整合三个强大的转换组装而成,如下所示:(i)不对称
-
Regioselective Synthesis of Substituted Carbazoles, Bicarbazoles, and Clausine C作者:Gary L. Points、Christopher M. BeaudryDOI:10.1021/acs.orglett.1c02449日期:2021.9.3Substituted carbazoles are efficiently constructed from 3-triflato-2-pyrones and alkynyl anilines. Multiple substituents are tolerated on the carbazole, and complete control of regiochemistry is observed. Complicated and sterically congested substitution patterns are produced. This strategy is also used to prepare substituted bicarbazoles and related biaryls. Finally, the method was showcased in a
-
Asymmetric Diels−Alder Reactions of 2-Pyrones with a Bifunctional Organic Catalyst作者:Yi Wang、Hongming Li、Yong-Qiang Wang、Yan Liu、Bruce M. Foxman、Li DengDOI:10.1021/ja070859h日期:2007.5.1The reactions of 2-pyrones with electron-deficient dienophiles constitute a synthetically useful class of Diels−Alder reaction. By exploring cinchona alkaloid-derived organic molecules as acid−base bifunctional catalysts, we successfully developed the first highly enantioselective and diastereoselective catalytic Diels−Alder reaction with 2-pyrones. Furthermore, we demonstrated the possibility of using
-
[EN] METHODS AND COMPOSITIONS FOR INHIBITION OF STAT3<br/>[FR] MÉTHODES ET COMPOSITIONS POUR L'INHIBITION DE STAT3申请人:OHIO STATE INNOVATION FOUNDATION公开号:WO2019067696A1公开(公告)日:2019-04-04In one aspect, the disclosure relates to prodrug compositions of a STAT inhibitor compound. In some aspects, the STAT is STAT3. Disclosed are pharmaceutical compositions comprising the prodrug inhibitors of STAT. In various aspects, the prodrug inhibitors of STAT can be used in methods of treating an inflammatory disorder, including multiple sclerosis, or a disorder of uncontrolled cellular proliferation, such as a cancer. This abstract is intended as a scanning tool for purposes of searching in the particular art and is not intended to be limiting of the present disclosure.
-
A novel small molecule LLL12B inhibits STAT3 signaling and sensitizes ovarian cancer cell to paclitaxel and cisplatin作者:Ruijie Zhang、Xiaozhi Yang、Dana M. Roque、Chenglong Li、Jiayuh LinDOI:10.1371/journal.pone.0240145日期:——
Ovarian cancer is the fifth most common cause of cancer deaths among American women. Platinum and taxane combination chemotherapy represents the first-line approach for ovarian cancer, but treatment success is often limited by chemoresistance. Therefore, it is necessary to find new drugs to sensitize ovarian cancer cells to chemotherapy. Persistent activation of Signal Transducer and Activator of Transcription 3 (STAT3) signaling plays an important role in oncogenesis. Using a novel approach called advanced multiple ligand simultaneous docking (AMLSD), we developed a novel nonpeptide small molecule, LLL12B, which targets the STAT3 pathway. In this study, LLL12B inhibited STAT3 phosphorylation (tyrosine 705) and the expression of its downstream targets, which are associated with cancer cell proliferation and survival. We showed that LLL12B also inhibits cell viability, migration, and proliferation in human ovarian cancer cells. LLL12B combined with either paclitaxel or with cisplatin demonstrated synergistic inhibitory effects relative to monotherapy in inhibiting cell viability and LLL12B-paclitaxel or LLL12B-cisplatin combination exhibited greater inhibitory effects than cisplatin-paclitaxel combination in ovarian cancer cells. Furthermore, LLL12B-paclitaxel or LLL12B-cisplatin combination showed more significant in inhibiting cell migration and growth than monotherapy in ovarian cancer cells. In summary, our results support the novel small molecule LLL12B as a potent STAT3 inhibitor in human ovarian cancer cells and suggest that LLL12B in combination with the current front-line chemotherapeutic drugs cisplatin and paclitaxel may represent a promising approach for ovarian cancer therapy.
卵巢癌是美国妇女癌症死亡的第五大原因。铂类和紫杉醇联合化疗代表了卵巢癌的一线治疗方法,但治疗成功通常受限于化疗耐药性。因此,有必要寻找新药物来增加卵巢癌细胞对化疗的敏感性。信号转导与转录激活因子3(STAT3)信号的持续激活在肿瘤发生中起着重要作用。使用一种称为高级多配体同时对接(AMLSD)的新方法,我们开发了一种新型非肽小分子LLL12B,它靶向STAT3通路。在这项研究中,LLL12B抑制了STAT3的磷酸化(酪氨酸705位点)以及其下游靶点的表达,这些靶点与癌细胞增殖和存活有关。我们展示了LLL12B还抑制了人类卵巢癌细胞的细胞存活能力、迁移和增殖。LLL12B与紫杉醇或顺铂结合相比于单药疗法在抑制细胞存活方面表现出协同抑制效果,LLL12B-紫杉醇或LLL12B-顺铂组合在卵巢癌细胞中的抑制效果比顺铂-紫杉醇组合更显著。此外,LLL12B-紫杉醇或LLL12B-顺铂组合在抑制细胞迁移和生长方面比单药疗法更显著。总之,我们的研究结果支持LLL12B作为一种有效的STAT3抑制剂在人类卵巢癌细胞中的作用,并建议LLL12B与当前一线化疗药物顺铂和紫杉醇的联合可能代表了一种有前途的卵巢癌治疗方法。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
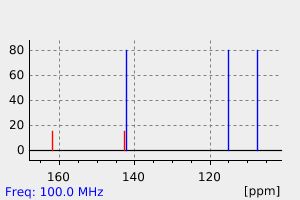
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息