4,4,4-三氟-1-(2-菲基)-1,3-丁烷二酮 | 15389-33-0
中文名称
4,4,4-三氟-1-(2-菲基)-1,3-丁烷二酮
中文别名
——
英文名称
4,4,4-Trifluoro-1-phenanthren-2-yl-butane-1,3-dione
英文别名
4,4,4-Trifluoro-1-(phenanthren-2-yl)butane-1,3-dione;4,4,4-trifluoro-1-phenanthren-2-ylbutane-1,3-dione
CAS
15389-33-0
化学式
C18H11F3O2
mdl
——
分子量
316.279
InChiKey
AQNQWKSQLGAUBI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):5.1
-
重原子数:23
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.11
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:5
安全信息
-
海关编码:2914700090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-乙酰基菲 2-acetylphenanthrene 5960-69-0 C16H12O 220.271
反应信息
-
作为反应物:描述:4,4,4-三氟-1-(2-菲基)-1,3-丁烷二酮 在 盐酸 、 platinum(IV) oxide 、 氢气 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 作用下, 以 四氢呋喃 、 乙醇 、 乙酸乙酯 为溶剂, 生成 2-氨基-N-[4-[5-(2-菲基)-3-(三氟甲基)-1H-吡唑-1-基]苯基]乙酰胺参考文献:名称:Development of novel antibacterial agents against methicillin-resistant Staphylococcus aureus摘要:Methicillin-resistant Staphylococcus aureus (MRSA) poses a serious threat to public health because of its resistance to multiple antibiotics most commonly used to treat infection. In this study, we report the unique ability of the cyclooxygenase-2 (COX-2) inhibitor celecoxib to kill Staphylococcus aureus and MRSA with modest potency. We hypothesize that the anti-Staphylococcus activity of celecoxib could be pharmacologically exploited to develop novel anti-MRSA agents with a distinct mechanism. Examination of an in-house, celecoxib-based focused compound library in conjunction with structural modifications led to the identification of compound 46 as the lead agent with high antibacterial potency against a panel of Staphylococcus pathogens and different strains of MRSA. Moreover, this killing effect is bacteria-specific, as human cancer cells are resistant to 46. In addition, a single intraperitoneal administration of compound 46 at 30 mg/kg improved the survival of MRSA-infected C57BL/6 mice. In light of its high potency in eradicating MRSA in vitro and its in vivo activity, compound 46 and its analogues warrant continued preclinical development as a potential therapeutic intervention against MRSA. (C) 2012 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2012.06.018
-
作为产物:参考文献:名称:Compounds and methods for inducing apoptosis in proliferating cells摘要:用于诱导增殖细胞凋亡的化合物,特别是癌细胞,包括但不限于前列腺癌、白血病、非小细胞肺癌、结肠癌、中枢神经系统癌、黑色素瘤、卵巢癌、肾癌、膀胱癌、淋巴瘤和乳腺癌。这些化合物在治疗雄激素非依赖性癌症特别有效,包括激素难治性前列腺癌。还提供了使用本发明化合物治疗患有癌症的受试者的方法。此外,还提供了使用本发明化合物治疗、抑制或延迟受试者癌症发生的方法。此外,还提供了使用本发明化合物诱导快速增殖细胞凋亡的方法,特别是虽然不一定是癌细胞。公开号:US20030236294A1
文献信息
-
MODIFICATION OF LAYERED SILICATES FOR LUMINESCENCE ACTIVATION申请人:Klauth Peter公开号:US20120107624A1公开(公告)日:2012-05-03The invention relates to a method for producing a luminescent layered silicate composite. The method according to the invention is characterized in that at least one luminescent dye, in particular fluorescent dye, on the basis of at least one complex, essentially a chelate complex, of at least one element of the rare earth elements (“rare earth complex”) is introduced between and/or stored in at least two layers of at least one layered silicate (“layered silicate layers”) respectively or that at least one luminescent dye, in particular fluorescent dye, on the basis of at least one complex, essentially a chelate complex, of at least one element of the rare earth elements (“rare earth complex”) is combined with a layered silicate to form a composite. The luminescent layered silicate composite according to the invention can be used for marking objects, for example plastic-based objects, or in the field of bioanalysis.
-
[EN] ELECTROLUMINESCENT MATERIALS AND DEVICES<br/>[FR] MATIERES ELECTROLUMINESCENTES ET DISPOSITIFS LES CONTENANT申请人:ELAM T LTD公开号:WO2004013252A1公开(公告)日:2004-02-12An electroluminescent device in which the electroluminescent layer comprises a rare earth, lanthanide or actinide bis[tris(pyrazolyl)borate)mono(acetonate).
表征谱图
-
氢谱1HNMR
-
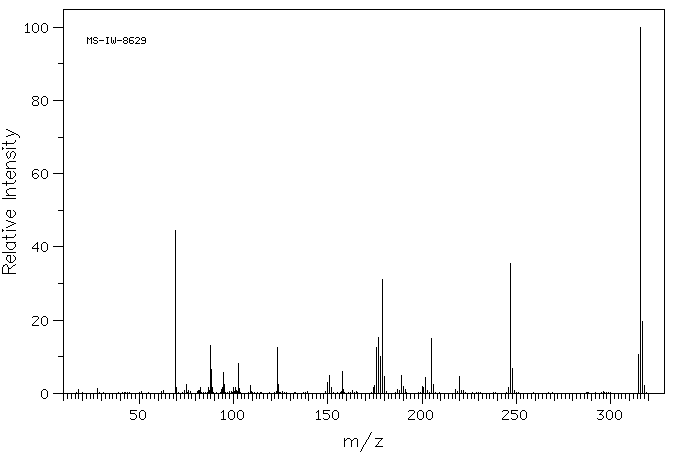
质谱MS
-
碳谱13CNMR
-
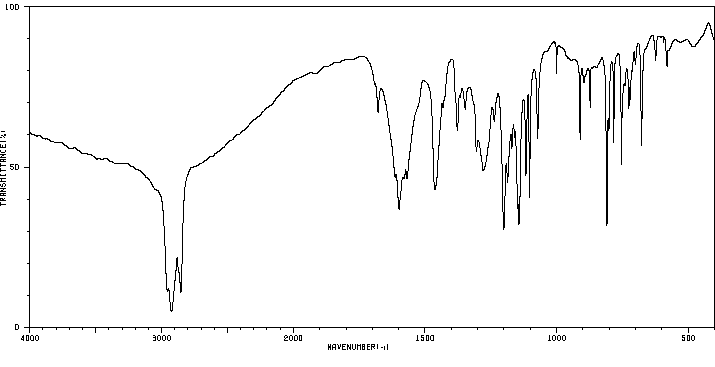
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(6,6)-苯基-C61己酸甲酯
高雌二醇
马兜铃酸钠
马兜铃酸盐
马兜铃酸C
马兜铃酸B
马兜铃酸(1:1MIXTUREOFARISTOLOCHICACIDIANDARISTOLOCHICACIDII)
马兜铃酸 Ia
马兜铃酸 IVa
马兜铃酸
颜料黑32
颜料红179
颜料红178
颜料红149
颜料红123
顺式-菲-1,2-二醇-3,4-环氧化物
顺式-苯并(a)屈-11,12-二醇-13,14-环氧化物
雷公藤酚A
镁二(1,4,5,6,7,16,17,18,19,19,20,20-十二氯六环[14.2.1.14,7.02,15.03,8.09,14]二十-5,9,11,13,17-五烯-11-磺酸酯)
钩大青酮
钩大青酮
钙(2+)12-羟基十八烷酸酯
酒石酸布托诺啡
那布扶林
还原红32
足球烯
贝那他汀B
贝母兰素
萘并[2,3-b]荧蒽
萘并[2,1-e][1]苯并二硫杂环戊烷
萘并[2,1-C:7,8-C']二菲
萘并[1,2-e][2]苯并呋喃-1,3-二酮
萘并[1,2-b]屈
萘并[1,2-a]蒽
萘并[1,2-B]菲-6-醇
萘二(六氯环戊二烯)加合物
萘,8-溴-1,2,3-三(1,1-二甲基乙基)-6-甲基-
菲醌单缩氨基硫脲
菲醌
菲并[9,10]呋喃
菲并[9,10-e]醋菲烯
菲并[4,5-bcd]噻吩
菲并[4,5-bcd]呋喃-3-醇
菲并[4,3-d]-1,3-二噁唑-5-羧酸,10-羟基-9-甲氧基-6-硝基-
菲并[3,2-b]噻吩
菲并[2,1-d]噻唑
菲并[2'',1'',10'':4,5,6;7'',8'',9'':4',5',6']二异喹啉并[2,1-a:2',1'-a']二萘嵌间二氮杂苯-8,13-二酮
菲并(3,4-b)噻吩
菲并(1,2-b)噻吩