4,5-二氨基-6-羟基嘧啶 | 1672-50-0
中文名称
4,5-二氨基-6-羟基嘧啶
中文别名
5,6-二氨基-4-嘧啶醇;5,6-二氨基-4(1H)-嘧啶酮;5,6-二氨基-4-羟基嘧啶
英文名称
4,5-diamino-6-hydroxypyrimidine
英文别名
4,5-Diamino-6-hydroxy-pyrimidin;5,6-diaminopyrimidin-4-ol;5,6-diamino-4-hydroxypyrimidine;5,6-Diamino-4-hydroxy-pyrimidin;4-hydroxy-5,6-diaminopyrimidine;4,5-Diaminohypoxanthine;4,5-diamino-1H-pyrimidin-6-one
CAS
1672-50-0
化学式
C4H6N4O
mdl
MFCD00006114
分子量
126.118
InChiKey
PWRHKLKFADDKHS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:239 °C
-
沸点:221.5±50.0 °C(Predicted)
-
密度:1.84
-
闪点:87.8℃
-
溶解度:可溶于水基(轻微)、DMSO(轻微)、水(非常轻微)
计算性质
-
辛醇/水分配系数(LogP):-1.7
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:93.5
-
氢给体数:3
-
氢受体数:4
安全信息
-
海关编码:29335990
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:存放于惰性气体中,避免接触空气。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 4,5-Diamino-6-hydroxypyrimidine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4,5-Diamino-6-hydroxypyrimidine
CAS number: 1672-50-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C4H6N4O
Molecular weight: 126.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 4,5-Diamino-6-hydroxypyrimidine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4,5-Diamino-6-hydroxypyrimidine
CAS number: 1672-50-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C4H6N4O
Molecular weight: 126.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4,5-二氨基-2-硫脲嘧啶 4,5-diamino-6-hydroxy-2-thiopyrimidine 1004-76-8 C4H6N4OS 158.184 5-硝基-6-氨基-4-羟基嘧啶 4-Amino-5-nitropyrimidin-6(1H)-on 36746-26-6 C4H4N4O3 156.101 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 6-氨基-5-甲酰氨基-3H-嘧啶-4-酮 6-amino-5-formylamino-3H-pyrimidin-4-one 64194-58-7 C5H6N4O2 154.128 N-(6-氨基-4-氧代-1H-嘧啶-5-基)乙酰胺 5-acetylamino-4-amino-6-oxo-1,6-dihydropyrimidine 50609-15-9 C6H8N4O2 168.155 —— 6-Amino-5-(benzylideneamino)-4-pyrimidinol 135039-36-0 C11H10N4O 214.227 —— 4-amino-5-hydroxyacetylamino-6-oxo-1,6-dihydropyrimidine 50609-14-8 C6H8N4O3 184.155 N-(6-氨基-4-氧代-1H-嘧啶-5-基)丙酰胺 4-amino-6-oxo-5-propanoylamino-1,6-dihydropyrimidine 92001-51-9 C7H10N4O2 182.182 —— (4-amino-6-oxo-1,6-dihydro-pyrimidin-5-ylimino)-acetic acid ethyl ester 108989-66-8 C8H10N4O3 210.192 N-(6-氨基-4-氧代-1H-嘧啶-5-基)-2-甲基丙酰胺 4-amino-5-(2-methylpropanoyl)amino-6-oxo-1,6-dihydropyrimidine 50609-12-6 C8H12N4O2 196.209
反应信息
-
作为反应物:描述:参考文献:名称:[EN] CONDENSED PYRIDINES AND PYRIMIDINES WITH TIE2 (TEK) ACTIVITY
[FR] PYRIDINES CONDENSEES ET PYRIMIDINES A ACTIVITE TIE2 (TEK)摘要:化合物的公式(I),其中A与其连接的碳原子一起形成一个融合的5-成员杂环芳烃环,其中所述的杂环芳烃环包含1个或2个从O、N和S中选择的杂原子,并且包含G的5-成员环与在公式(I)中标记为桥头碳#的A形成的环在间位连接;G从O、S和NR5中选择;Z从N和CR6中选择;Q1从可选择的取代芳基和杂环芳基中选择,取代基R1到R6如文本中所定义,用于在温血动物(如人)中产生抗血管生成作用。公开号:WO2004013141A1 -
作为产物:描述:4,5-Diamino-6-hydroxypyrimidine sulphate 在 polymer-supported carbonate 作用下, 以 N,N-二甲基甲酰胺 、 乙腈 为溶剂, 反应 24.0h, 生成 4,5-二氨基-6-羟基嘧啶参考文献:名称:WO2007/71966摘要:公开号:
文献信息
-
[EN] SMALL MOLECULE INHIBITORS OF NF-kB INDUCING KINASE<br/>[FR] INHIBITEURS À PETITE MOLÉCULE DE KINASE INDUISANT NF-KB申请人:JANSSEN PHARMACEUTICA NV公开号:WO2020239999A1公开(公告)日:2020-12-03The present invention relates to compounds that inhibit NIK and pharmaceutical compositions comprising such compounds and methods of using the same. These compounds and pharmaceutical compositions are envisaged to be useful for preventing or treating diseases such as cancer (such as B-cell malignancies including leukemias, lymphomas and myeloma), inflammatory disorders, autoimmune disorders, immunodermatologic disorders such as palmoplantar pustulosis and hidradenitis suppurativa, and metabolic disorders such as obesity and diabetes.本发明涉及抑制NIK的化合物,以及包含这些化合物的药物组合物和使用方法。这些化合物和药物组合物预期能用于预防或治疗癌症(如包括白血病、淋巴瘤和多发性骨髓瘤的B细胞恶性肿瘤)、炎症性疾病、自身免疫疾病、免疫皮肤病学疾病(如掌跖脓疱病和化脓性汗腺炎)以及代谢紊乱疾病(如肥胖和糖尿病)。
-
Synthesis and Biological Evaluation of New Dipyridylpteridines, Lumazines, and Related Analogues作者:Zina A. A. Abbas、Najwa M. J. Abu-Mejdad、Zeenah W. Atwan、Najim A. Al-MasoudiDOI:10.1002/jhet.2651日期:2017.3methanolic ammonia under drastic conditions. Condensation of 2 or 29 with 2‐oxo‐2‐(thiophen‐2‐yl)acetaldehyde oxime (11) gave the 6‐(2‐thienyl)‐pteridine‐4‐one (12) and 5‐chloro‐2‐(2‐thienyl)pyrido[3,4‐b]pyrazine (31), respectively. All compounds were evaluated for their antiviral activity against the replication of HIV‐1 and HIV‐2 in MT‐4. Some of the synthesized compounds were tested against the bacterial4,5-二氨基嘧啶2和3与2,2ʹ-联吡啶(4)的缩合分别得到6,7-双(2-吡啶基)蝶啶-2-一类似物5和7。类似地,6,7-双(2-吡啶基)luamzine衍生物13,15,17,和23,从5,6-二氨基-2- thiopyrimidines反应合成13,14,和22与4,分别,而缩合4,5,6-三氨基嘧啶(25)或5,6-二氨基类似物26与4分别提供了4-氨基蝶啶类似物27和28。得到4-硫代类似物的新蝶啶和lumazines的硫杂化6,8,16,和24。用甲醇氨水处理6和8分别得到4-异is呤类似物9和10。用取代的苯甲酰氯将15烷基化,得到18和19,它们环化为噻唑并蝶啶衍生物20和21分别用多磷酸处理。或者,在剧烈条件下,用甲醇氨处理24,制得27。2或29与2-氧代-2-(噻吩-2-基)乙醛肟(11)的缩合得到6-(2-噻吩基)-哌啶-4-基(12)和5-氯-2-(- 2-噻吩基]吡啶并[3
-
[EN] QUINUCLIDINE COMPOUNDS AS ALPHA-7 NICOTINIC ACETYLCHOLINE RECEPTOR LIGANDS<br/>[FR] COMPOSÉ DE QUINUCLIDINE COMME LIGANDS DU RÉCEPTEUR NICOTINIQUE ALPHA-7 DE L'ACÉTYLCHOLINE申请人:BRISTOL MYERS SQUIBB CO公开号:WO2011053292A1公开(公告)日:2011-05-05The disclosure provides compounds of formula I, including their salts, as well as compositions and methods of using the compounds. The compounds are ligands for the nicotinic 7 receptor and may be useful for the treatment of various disorders of the central nervous system, especially affective and neurodegenerative disorders.
-
QUINUCLIDINE COMPOUNDS AS ALPHA-7 NICOTINIC ACETYLCHOLINE RECEPTOR LIGANDS申请人:Cook, II James H.公开号:US20090270405A1公开(公告)日:2009-10-29The disclosure provides compounds of formula I, including their salts, as well as compositions and methods of using the compounds. The compounds are ligands for the nicotinic α7 receptor and may be useful for the treatment of various disorders of the central nervous system, especially affective and neurodegenerative disorders.
-
Pyrimidines. Part XXIII. Synthesis of pyrimido[4,5-b][1,4]oxazines by reaction of 4,5-diaminopyrimidine derivatives with α-halogeno-ketones作者:Jean Mirza、Wolfgang Pfleiderer、A. D. Brewer、Alexander Stuart、H. C. S. WoodDOI:10.1039/j39700000437日期:——ketone is shown to give pyrimido [4,5-b][1,4]oxazines and not the expected dihydropteridine derivatives. Similar condensations with a number of different α-halogeno-aldehydes and α-halogeno-ketones have been studied, and the factors which control the nature of the product have been determined. Some preliminary studies of the chemical reactivity of pyrimido-[4,5-b][1,4]oxazines have been carried out.
表征谱图
-
氢谱1HNMR
-
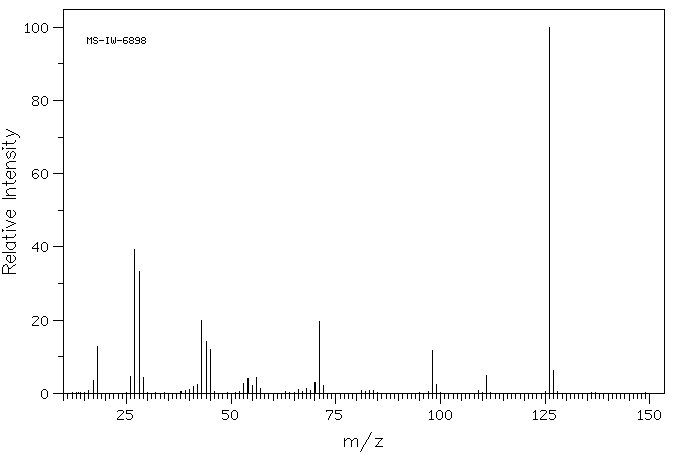
质谱MS
-
碳谱13CNMR
-
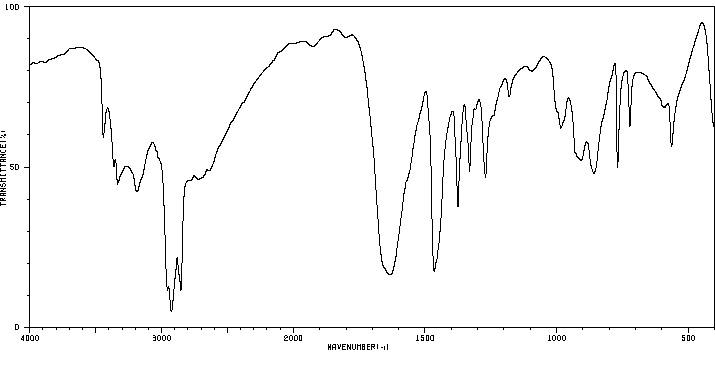
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3