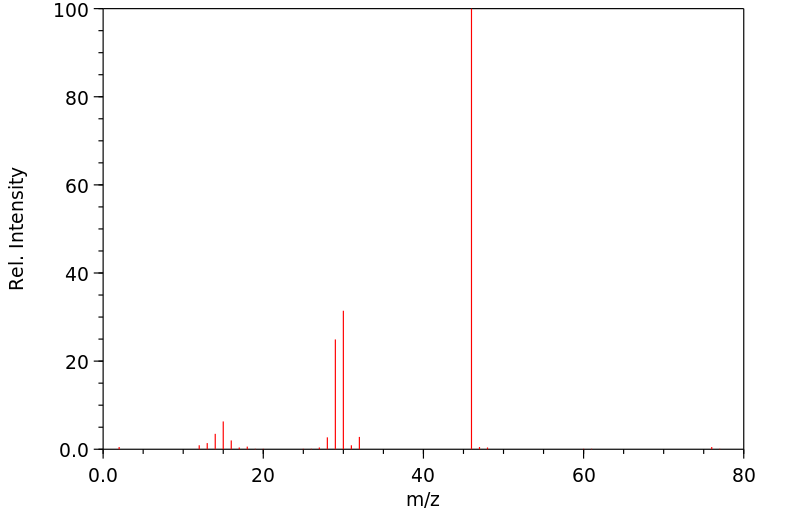
毒理性
Methemoglobinemia - The presence of increased methemoglobin in the blood; the compound is classified as secondary toxic effect
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases