4-(5)-硝基茚满 | 7436-07-9
中文名称
4-(5)-硝基茚满
中文别名
5-硝基茚满
英文名称
5-nitroindane
英文别名
5-Nitro-indane;5-nitro-2,3-dihydro-1H-indene;5-Nitro-indan;2,3-dihydro-5-nitro-1H-indene;5-Nitroindan
CAS
7436-07-9
化学式
C9H9NO2
mdl
MFCD00003801
分子量
163.176
InChiKey
DLURUQQMVLOLCP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:40.25°C
-
沸点:290.25°C (rough estimate)
-
密度:1.2021 (rough estimate)
-
保留指数:261
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
SDS
| Name: | 5-Nitroindan 99% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 7436-07-9 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 7436-07-9 | 5-Nitroindan, 99% | 99 | 231-088-0 |
Risk Phrases:
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower lids.
Skin:
Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do NOT induce vomiting. Allow the victim to rinse his mouth and then to drink 2-4 cupfuls of water, and seek medical advice.
Inhalation:
Remove from exposure to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Autoignition Temperature: Not available.
Flash Point: Not available.
NFPA Rating: Not published.
Explosion Limits, Lower: Not available.
Upper: Not available.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material, (e.g., dry sand or earth), then place into a chemical waste container. Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Personal Protective Equipment Eyes: Wear safety glasses and chemical goggles if splashing is possible.
Skin:
Wear appropriate protective gloves and clothing to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Wear a NIOSH/MSHA-approved (or equivalent) full-facepiece airline respirator in the positive pressure mode with emergency escape provisions.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Not available.
Appearance: brown green
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Vapor Density: Not available.
Evaporation Rate: Not available.
Viscosity: Not available.
Boiling Point: @ 760.00mm Hg
Freezing/Melting Point: 39.00 - 40.00 deg C
Decomposition Temperature: Not available.
Solubility: Not available.
Specific Gravity/Density: Not available.
Molecular Formula: C9H9NO2
Molecular Weight: 163.18
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 7436-07-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
5-Nitroindan, 99% - Not listed by ACGIH, IARC, NIOSH, NTP, or OSHA.
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
For further information, contact Fisher Scientific.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
CDG/CPL
IMO
Not regulated as a hazardous material.
IATA
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Canadian TDG
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 7436-07-9:
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
WHMIS: Not available.
CAS# 7436-07-9 is not listed on Canada's Ingredient Disclosure List.
Exposure Limits
US FEDERAL
TSCA
CAS# 7436-07-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 6-硝基-1-茚满酮 6-nitroindan-1-one 24623-24-3 C9H7NO3 177.159 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-硝基茚满酮 5-nitroindanone 22246-24-8 C9H7NO3 177.159 5-氨基茚旦 5-Aminoindan 24425-40-9 C9H11N 133.193 —— 1,1,3,3-tetrachloro-5-nitroindan 76213-19-9 C9H5Cl4NO2 300.956 N1-(2,3-二氢-1H-茚-5-基)乙酰胺 5-acetamidoindane 59856-06-3 C11H13NO 175.23
反应信息
-
作为反应物:描述:参考文献:名称:Kolodkina,I.I. et al., Journal of general chemistry of the USSR, 1963, vol. 33, p. 461 - 465摘要:DOI:
-
作为产物:描述:参考文献:名称:Gibbs,H.W. et al., Journal of the Chemical Society. Perkin transactions II, 1978, p. 1145 - 1157摘要:DOI:
文献信息
-
Dichlorine monoxide: a powerful and selective chlorinating reagent作者:F. D. Marsh、W. B. Farnham、D. J. Sam、B. E. SmartDOI:10.1021/ja00381a032日期:1982.8-Dichlorine monoxide is a powerful and selective reagent for either side chain or ring chlorination of deactivated aromatic substrates. It gives excellent product yields under mild conditions where conventional reagents fail or require harsh conditions. The mechanistic aspects of the dichlorine monoxide reactions have been probed using linear free-energy relationships, isotope effects and competitive
-
Polymethylhydrosiloxane derived palladium nanoparticles for chemo- and regioselective hydrogenation of aliphatic and aromatic nitro compounds in water作者:Dandu Damodara、Racha Arundhathi、T. Venkata Ramesh Babu、Margaret K. Legan、Hephzibah J. Kumpaty、Pravin R. LikharDOI:10.1039/c4ra01333f日期:——unsaturated, aromatic and heteroaromatic nitro compounds into their corresponding amines has been achieved with highly efficient polysiloxane-stabilised “Pd” nanoparticles on NAP-magnesium oxide supports using an environmentally friendly hydrogenating agent, polymethylhydrosiloxane [PMHS] in water. Highly stable and active Pd nanoparticles were prepared by the reduction of NAP-Mg-PdCl4 with PMHS, which serves
-
NOVEL COMPOUNDS AS DIACYLGLYCEROL ACYLTRANSFERASE INHIBITORS申请人:GlaxoSmithKline LLC公开号:US20140148437A1公开(公告)日:2014-05-29This invention relates to novel compounds which are inhibitors of acyl coenzyme A: diacylglycerol acyltransferase 1 (DGAT-1), to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy, alone or in combination with weight management therapies or other triglyceride lowering therapy for the prevention or treatment of diseases related to DGAT-1 dysfunction or where modulation of DGAT-1 activity may have therapeutic benefit including but not limited to obesity, obesity related disorders, genetic (Type 1, Type 5 hyperlipidemia) and acquired forms of hypertriglyceridemia or hyperlipoproteinemia-related disorders, caused by but not limited to lipodystrophy, hypothyroidism, medications (beta blockers, thiazides, estrogen, glucocorticoids, transplant) and other factors (pregnancy, alcohol intake), hyperlipoproteinemia, chylomicronemia, dyslipidemia, non-alcoholic steatohepatitis, diabetes, insulin resistance, metabolic syndrome, cardiovascular outcomes, angina, excess hair growth (including syndromes associated with hirsutism), nephrotic syndrome, fibrosis such as myocardial, renal and liver fibrosis, hepatitis C virus infection and acne or other skin disorders.这项发明涉及一种新型化合物,它们是酰辅酶A:二酰基甘油酰基转移酶1(DGAT-1)的抑制剂,以及含有它们的药物组合物,它们的制备方法,以及它们在治疗中的使用,单独或与体重管理疗法或其他降甘油三酯疗法结合,用于预防或治疗与DGAT-1功能障碍相关的疾病,或者调节DGAT-1活性可能具有治疗益处的疾病,包括但不限于肥胖、与肥胖相关的疾病、遗传性(1型、5型高脂血症)和获得性高三酰甘油脂或高脂蛋白血症相关疾病,由脂肪萎缩、甲状腺功能减退、药物(β受体阻滞剂、噻嗪类药物、雌激素、糖皮质激素、移植)和其他因素(怀孕、饮酒)引起,高脂蛋白血症、乳糜微粒血症、血脂异常、非酒精性脂肪肝炎、糖尿病、胰岛素抵抗、代谢综合征、心血管结果、心绞痛、多毛(包括与多毛症相关的综合征)、肾病综合征、纤维化如心肌、肾脏和肝脏纤维化、丙型肝炎病毒感染和痤疮或其他皮肤疾病。
-
Imidazole derivatives申请人:Hoffmann-La Roche Inc.公开号:US04435406A1公开(公告)日:1984-03-06Tricyclic imidazole derivatives of the formula ##STR1## wherein R.sup.1 is 2-pyridyl optionally substituted by lower alkyl or lower alkoxy, n is the integer 0 or 1, R.sup.2 is hydrogen or lower alkyl, R.sup.3 and R.sup.4, independently, are hydrogen or lower alkyl, A is a group of the formula ##STR2## m is the integer 2 or 3, R.sup.5, R.sup.6, R.sup.7 and R.sup.8, independently, are hydrogen or lower alkyl, and R.sup.9 is hydrogen and R.sup.10 is hydrogen or lower alkyl or R.sup.9 and R.sup.10 taken together are oxo, provided that at least one of R.sup.3 and R.sup.4 is lower alkyl when A is a group of the formula --CH.dbd.CH--CH.dbd.CH-- or --(CH.sub.2).sub.4 --, and their pharmaceutically acceptable acid addition salts. The compounds of formula I inhibit gastric acid secretion and prevent the formation of gastric ulcers.Tricyclic imidazole derivatives of the formula ##STR1## wherein R.sup.1 is 2-pyridyl optionally substituted by lower alkyl or lower alkoxy, n is the integer 0 or 1, R.sup.2 is hydrogen or lower alkyl, R.sup.3 and R.sup.4, independently, are hydrogen or lower alkyl, A is a group of the formula ##STR2## m is the integer 2 or 3, R.sup.5, R.sup.6, R.sup.7 and R.sup.8, independently, are hydrogen or lower alkyl, and R.sup.9 is hydrogen and R.sup.10 is hydrogen or lower alkyl or R.sup.9 and R.sup.10 taken together are oxo, provided that at least one of R.sup.3 and R.sup.4 is lower alkyl when A is a group of the formula --CH.dbd.CH--CH.dbd.CH-- or --(CH.sub.2).sub.4 --, and their pharmaceutically acceptable acid addition salts. The compounds of formula I inhibit gastric acid secretion and prevent the formation of gastric ulcers.
-
Copper-Catalyzed Cross-Coupling of Benzylic C–H Bonds and Azoles with Controlled <i>N</i>-Site Selectivity作者:Si-Jie Chen、Dung L. Golden、Shane W. Krska、Shannon S. StahlDOI:10.1021/jacs.1c07117日期:2021.9.15ambident reactivity of many azoles, however, presents significant selectivity challenges. Here, we report a copper-catalyzed method that achieves site-selective cross-coupling of pyrazoles and other N–H heterocycles with substrates bearing (hetero)benzylic C–H bonds. Excellent N-site selectivity is achieved, with the preferred site controlled by the identity of co-catalytic additives. This cross-coupling
表征谱图
-
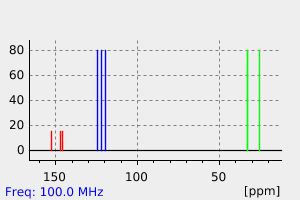
氢谱1HNMR
-
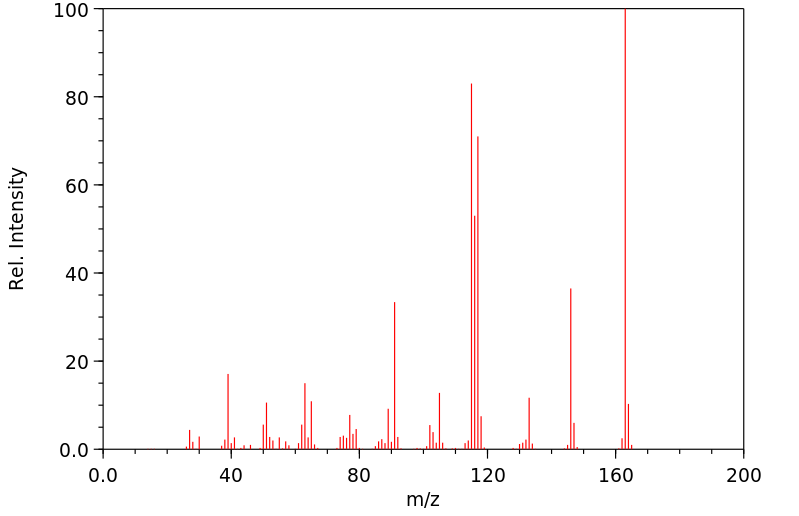
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-7,7-双[(4S)-(苯基)恶唑-2-基)]-2,2,3,3-四氢-1,1-螺双茚满
(R)-7,7-双[(4S)-(苯基)恶唑-2-基)]-2,2,3,3-四氢-1,1-螺双茚满
(4S,5R)-3,3a,8,8a-四氢茚并[1,2-d]-1,2,3-氧杂噻唑-2,2-二氧化物-3-羧酸叔丁酯
(3aS,8aR)-2-(吡啶-2-基)-8,8a-二氢-3aH-茚并[1,2-d]恶唑
(3aS,3''aS,8aR,8''aR)-2,2''-环戊二烯双[3a,8a-二氢-8H-茚并[1,2-d]恶唑]
(1α,1'R,4β)-4-甲氧基-5''-甲基-6'-[5-(1-丙炔基-1)-3-吡啶基]双螺[环己烷-1,2'-[2H]indene
齐洛那平
鼠完
麝香
风铃醇
颜料黄138
顺式-1,6-二甲基-3-(4-甲基苯基)茚满
雷美替胺杂质9
雷美替胺杂质24
雷美替胺杂质14
雷美替胺杂质13
雷美替胺杂质10
雷美替胺杂质
雷美替胺杂质
雷美替胺杂质
雷美替胺杂质
雷美替胺杂质
雷美替胺
雷沙吉兰相关化合物HCl
雷沙吉兰杂质8
雷沙吉兰杂质5
雷沙吉兰杂质4
雷沙吉兰杂质3
雷沙吉兰杂质16
雷沙吉兰杂质15
雷沙吉兰杂质12
雷沙吉兰杂质1
雷沙吉兰杂质
雷沙吉兰13C3盐酸盐
雷沙吉兰
阿替美唑盐酸盐
铵2-(1,3-二氧代-2,3-二氢-1H-茚-2-基)-8-甲基-6-喹啉磺酸酯
金粉蕨辛
金粉蕨亭
重氮正癸烷
酸性黄3[CI47005]
酒石酸雷沙吉兰
还原茚三酮(二水)
还原茚三酮
过氧化,2,3-二氢-1H-茚-1-基1,1-二甲基乙基
贝沙罗汀杂质8
表蕨素L
螺双茚满
螺[茚-2,4-哌啶]-1(3H)-酮盐酸盐
螺[茚-2,4'-哌啶]-1(3H)-酮