磷酸三丁酯 | 126-73-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-79 °C (lit.)
-
沸点:180-183 °C/22 mmHg (lit.)
-
密度:0.979 g/mL at 25 °C (lit.)
-
蒸气密度:9.2 (vs air)
-
闪点:380 °F
-
溶解度:水:1ml溶于165ml水
-
介电常数:6.5(20℃)
-
暴露限值:TLV-TWA 2.18 mg/m3 (0.2 ppm) (ACGIH), 5 mg/m3 (OSHA and NIOSH); IDLH 1300 mg/m3 (120 ppm) (NIOSH).
-
LogP:4 at 20℃
-
物理描述:Tributyl phosphate is an odorless colorless to yellow liquid. Toxic by ingestion and inhalation.
-
颜色/状态:Colorless to pale-yellow liquid
-
气味:Odorless
-
蒸汽密度:9.2 (NTP, 1992) (Relative to Air)
-
蒸汽压力:1.13X10-3 mm Hg at 25 °C
-
稳定性/保质期:
-
自燃温度:410 °C
-
分解:289 °C
-
粘度:3.39 cP at 25 °C
-
燃烧热:-1905.7 kcal/mol at 25 °C
-
汽化热:55.1 cal/g at 289 °C
-
表面张力:27.55 dyne/cm at 20 °C
-
气味阈值:Odor perception threshold 0.014 mg/L.
-
折光率:Index of refraction = 1.4224 at 25 °C
-
保留指数:1613;1617;1622;1616;1623;1636;1615.7;1623;1619;1612;1612;1613;1619;1663;1621;1615;1620;1614;1616;1613.3;283.2;278.79
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:17
-
可旋转键数:12
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:44.8
-
氢给体数:0
-
氢受体数:4
ADMET
安全信息
-
职业暴露等级:B
-
职业暴露限值:TWA: 0.2 ppm (2.5 mg/m3)
-
TSCA:Yes
-
立即威胁生命和健康浓度:30 ppm
-
危险品标志:Xn,F
-
安全说明:S16,S36/37,S36/37/39,S45,S46,S53
-
危险类别码:R38,R40,R36/37/38,R22,R20/22,R11,R48/20,R62
-
WGK Germany:2
-
海关编码:2919900090
-
危险品运输编号:UN 1208 3/PG 2
-
危险类别:6.1
-
RTECS号:TC7700000
-
包装等级:I; II; III
-
储存条件:存储于阴凉通风处,并按照有毒化学品的规定进行运输和储存。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 磷酸三丁酯 |
| 化学品英文名称: | Tributyl phosphate |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 126-73-8 |
| 分子式: | C 12 H 27 O 4 P |
| 分子量: | 266.32 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | |||
| 化学品名称:磷酸三丁酯 | |||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 |
| 健康危害: | 本品对人血、血浆中胆碱酯酶有轻度抑制作用。人经口,约100ml,可引起呼吸困难、抽搐、麻痹、昏睡等症状。对皮肤有刺激作用。蒸气和烟雾对眼睛、粘膜和上呼吸道有刺激作用。接触后可引起中枢神经系统的刺激症状。 |
| 环境危害: | |
| 燃爆危险: |
| 第四部分:急救措施 |
| 皮肤接触: | 脱去污染的衣着,用肥皂水及清水彻底冲洗。 |
| 眼睛接触: | 立即翻开上下眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 脱离现场至空气新鲜处。呼吸困难时给输氧;呼吸停止时,立即进行人工呼吸。就医。 |
| 食入: | 误服者立即漱口,给饮牛奶或蛋清,催吐,洗胃。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇高热、明火或与氧化剂接触,有引起燃烧的危险。受热分解产生剧毒的氧化磷烟气。 |
| 有害燃烧产物: | |
| 灭火方法及灭火剂: | 泡沫、二氧化碳、干粉、砂土。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | |
| 闪点(℃): | 146 |
| 自燃温度(℃): | 引燃温度(℃):410 |
| 爆炸下限[%(V/V)]: | 无资料 |
| 爆炸上限[%(V/V)]: | 无资料 |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 疏散泄漏污染区人员至安全区,禁止无关人员进入污染区,切断火源。应急处理人员戴好防毒面具,穿防护服。在确保安全情况下堵漏。用大量水冲洗,经稀释的洗液放入废水系统。如大量泄漏,利用围堤收容,然后收集、转移、回收或无害处理后废弃。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | |
| 储存注意事项: |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联MAC:0.5mg/m3 美国TLV—TWA:2.5mg/ |
| 监测方法: | |
| 工程控制: | 生产过程密闭,加强通风。 |
| 呼吸系统防护: | 空气中浓度超标时,戴面具式呼吸器。紧急事态抢救或撤离时,佩带自给式呼吸器。 |
| 眼睛防护: | 戴化学安全防护眼镜。 |
| 身体防护: | 穿工作服。 |
| 手防护: | 戴橡皮胶手套。 |
| 其他防护: | 工作现场禁止吸烟、进食和饮水。工作后,淋浴更衣。保持良好的卫生习惯。 |
| 第九部分:理化特性 |
| 外观与性状: | 无色、无味粘稠液体。 |
| pH: | |
| 熔点(℃): | <-79 |
| 沸点(℃): | 180-183(2.87kPa) |
| 相对密度(水=1): | 0.98 |
| 相对蒸气密度(空气=1): | 7.67 |
| 饱和蒸气压(kPa): | 2.67(20℃) |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | 146 |
| 引燃温度(℃): | 引燃温度(℃):410 |
| 爆炸上限%(V/V): | 无资料 |
| 爆炸下限%(V/V): | 无资料 |
| 分子式: | C 12 H 27 O 4 P |
| 分子量: | 266.32 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 溶于水,溶于多数有机溶剂。 |
| 主要用途: | 用作溶剂,还常作为硝基纤维素、醋酸纤维素、氯化橡胶和聚氯乙烯的增塑剂,稀有金属的萃取剂等,也用作热交换介质。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂、强酸、强碱。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳、氧化磷。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | 对皮肤和呼吸道有强烈的刺激作用,具有全身致毒作用。 LD50:3000mg/kg(大鼠经口) LC50:实验大鼠(三只)吸入1.3g/m3,6小时,无死亡。 |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | |
| UN编号: | |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 储存于阴凉、通风仓间内。远离火种、热源。保持容器密封。应与氧化剂、酸类、碱类分开存放。搬运时要轻装轻卸,防止包装及容器损坏。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 4 |
| MSDS修改日期: | 年月日 |
制备方法与用途
磷酸三丁酯(简称TBP)是一种无色无臭的液体,分子式为C₁₂H₂₇PO₄,结构式为(C₄H₉O)₃P=O。其熔点为-80℃,沸点为289℃(分解),相对密度介于0.973~0.978(20/4℃),折光率为1.4215(25℃)。这种物质对光稳定,能与多种有机溶剂混溶,并难溶于水。TBP由正丁醇和三氯氧磷进行酯化制得。
磷酸三丁酯是典型的中性络合萃取剂,性质稳定,耐强酸、强碱、强氧化剂和强辐射,在核燃料和冶金工业中得到广泛应用。它是全世界萃取剂产量中吨位最大的产品之一,主要用于从硝酸溶液中萃取铀,对铀的提取起着至关重要的作用。此外,它还常作为硝基纤维素、醋酸纤维素、氯化橡胶和聚氯乙烯的增塑剂,能使这些制品具有耐寒性和耐光性。
化学性质磷酸三丁酯是一种无色、有刺激性气味的透明液体,闪点为193℃,沸点为289℃(101KPa),粘度介于3.5~12.2厘泊,折光率为1.4226(20℃)。它能与通常的有机溶剂混溶,并稍溶于水,水在本品中的溶解度约为7%(25℃)。随着温度上升,会逐渐发生水解。
磷酸三丁酯主要用作溶剂,还常作为硝基纤维素、醋酸纤维素、氯化橡胶和聚氯乙烯的增塑剂。它的沸点低且挥发损失较大,在增塑剂上的应用受到限制。此外,它还可用于萃取铀、钍、钒等稀有金属,并在一些树脂中用作阻燃剂。
用途磷酸三丁酯用作金属络合物的萃取剂,硝酸纤维素、醋酸纤维素、氯化橡胶和聚氯乙烯的增塑剂,涂料、油墨和粘合剂的溶剂。此外,它对丙烯酸酯类、甲基丙烯酸酯类、丙烯酸、丙烯腈、苯乙烯、丁二烯有较好的阻聚效果。
磷酸三丁酯还用作硝化纤维素、乙酸纤维素、氯化橡胶和聚氯乙烯的主增塑剂,也常用作涂料、粘合剂和油墨的溶剂、消泡剂、消静电剂以及稀土元素的萃取剂。其大鼠LD₅₀值为3000毫克/公斤。
磷酸三丁酯还可用作气相色谱固定液、硝化纤维和乙基纤维素的溶剂、增塑剂及有机合成中间体,用于萃取钴、铱、锰、钼、钯、铑、镍、铀和钨,并可用于比色测定钼。此外,它还可作为稀土金属分离用剂。
生产方法磷酸三丁酯由正丁醇与三氯氧磷反应制得。每吨原料消耗:正丁醇1500公斤,三氯氧磷770公斤。
类别与毒性磷酸三丁酯是一种有毒物质,属于中毒级别。其急性口服毒性(大鼠)LD₅₀为3000毫克/公斤;小鼠的LD₅₀为1189毫克/公斤。
皮肤接触:兔实验中,10毫克/24小时无明显刺激反应;眼睛接触500毫克会出现重度刺激。
磷酸三丁酯可燃,在火场中会释放有毒磷氧化物烟雾。库房应低温、通风并保持干燥。
职业暴露标准:时间加权平均容许浓度(TWA)和短时间接触极限(STEL)均为5毫克/立方米。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 磷酸二丁酯 dibutyl phosphate 107-66-4 C8H19O4P 210.21 亚磷酸二丁酯 dibutyl hydrogen phosphite 1809-19-4 C8H19O3P 194.211 三丁基,三丁酯 tri-n-butyl phosphite 102-85-2 C12H27O3P 250.318 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— phosphoric acid dibutyl ester ethyl ester 7242-58-2 C10H23O4P 238.264 丁基二乙基磷酸酯 n-butyl diethyl phosphate 2737-00-0 C8H19O4P 210.21 磷酸二丁酯 dibutyl phosphate 107-66-4 C8H19O4P 210.21 —— Dibutyl 4-hydroxybutyl phosphate 89197-75-1 C12H27O5P 282.31 —— phosphoric acid dibutyl ester-octyl ester 25786-28-1 C16H35O4P 322.425 磷酸单丁酯 n-butyl phosphoric acid 1623-15-0 C4H11O4P 154.103
反应信息
-
作为反应物:描述:磷酸三丁酯 在 salophen(tBu)AlBr 作用下, 以 chloroform-d1 为溶剂, 反应 24.0h, 以100%的产率得到正溴丁烷参考文献:名称:Cleavage of phosphate ester bonds by use of novel group 13 chelate compounds摘要:一种新的化合物具有通用公式(LX)nY,其中X从除硼以外的第13族元素组中选择,Y从卤素、氯酸盐、硫酸盐和硝酸盐组中选择,L是含有两个氮和两个氧供体基团的螯合配体,其中n=1或2。公开号:US07105703B1
-
作为产物:描述:参考文献:名称:A General Method for the Conversion of Thiophosphoryl and Selenophosphoryl Groups into Phosphoryl Groups by Ozone Oxidation摘要:DOI:10.1055/s-1983-30407
-
作为试剂:描述:2,6-二甲基吡啶 、 1,3-二溴-5,5-二甲基海因 在 copper(l) iodide 、 磷酸三丁酯 、 potassium carbonate 、 三苯基膦 作用下, 以 甲基叔丁基醚 为溶剂, 反应 24.0h, 以43%的产率得到参考文献:名称:一种N-(芳基/杂芳基)烷基-二酰胺的制备方 法摘要:本发明涉及一种N‑(芳基/杂芳基)烷基‑二酰胺的制备方法,在氮气保护下,将过渡金属、膦或氮配体、助催化剂、碱、溶剂、N‑卤代环二酰胺、烷基‑芳环或烷基‑杂芳环化合物依次加入到反应容器中,于80~140℃发生氧化胺化反应,6~48小时后反应结束,经蒸干溶剂、柱层析分离即得N‑(芳基/杂芳基)烷基‑二酰胺类化合物。本发明合成工艺简单,反应条件温和,产率高,易于工业化。公开号:CN112047925B
文献信息
-
Regio- and Stereoselective (SN2) N-, O-, C- and S-Alkylation Using Trialkyl Phosphates作者:Amit Banerjee、Tomohiro Hattori、Hisashi YamamotoDOI:10.1055/a-1504-8366日期:2023.1Bimolecular nucleophilic substitution (SN2) is one of the most well-known fundamental reactions in organic chemistry to generate new molecules from two molecules. In principle, a nucleophile attacks from the back side of an alkylating agent having a suitable leaving group, most commonly a halide. However, alkyl halides are expensive, very harmful, toxic and not so stable, which makes them problematic双分子亲核取代 (SN2) 是有机化学中最著名的基本反应之一,用于从两个分子生成新分子。原则上,亲核试剂从具有合适离去基团(最常见的是卤化物)的烷化剂的背面攻击。然而,烷基卤价格昂贵、非常有害、有毒且不稳定,这使得它们在实验室使用中存在问题。相比之下,磷酸三烷基酯价格低廉、易于获得且在室温、空气中稳定且易于处理,但很少用作有机合成中的烷基化剂。在这里,我们描述了一种使用现成的磷酸三烷基酯对各种 N-、O-、C- 和 S-亲核试剂进行亲核烷基化的温和、直接和强大的方法。反应以优异的收率顺利进行,和定量产量在许多情况下,并涵盖广泛的底物。此外,通过手性中心构型的反转(高达 98% ee)实现了仲烷基的罕见立体选择性转移。
-
SYNTHESIS OF 1,1,2,3-TETRACHLOROPROPENE申请人:Honeywell International Inc.公开号:US20140221705A1公开(公告)日:2014-08-07The present invention provides an improved process for producing 1,1,2,3-tetrachloropropene. By using a first reactive distillation column for HCC-250fb dehydrochlorination, and a second reactive distillation column for HCC-240db dehydrochlorination/HCC-1230xf isomerization, the 1,1,2,3-tetrachloropropene manufacturing process can be greatly simplified, resulting in reduced equipment use, energy use, as well as increased productivity.
-
SULFONIUM COMPOUND, PHOTO-ACID GENERATOR, AND METHOD FOR MANUFACTURING THE SAME申请人:JOO Hyun Sang公开号:US20120172606A1公开(公告)日:2012-07-05A sulfonium compound represented by formula (1), a photo-acid generator, and a method for producing a sulfonium compound are provided: wherein X represents an electron-donating group; R 1 and R 2 each represent an alkyl group, a cycloalkyl group, or the like; R 3 and R 4 each represent an arylene group or a heteroarylene group; R 5 and R 6 each represent an alkyl group, a cycloalkyl group, or the like; and A − and B − are anions that are different from each other. The sulfonium compound, when used as a photo-acid generator, can produce a uniform and excellent resist pattern.提供一个由公式(1)表示的磺onium化合物,一种光酸发生器,以及一种生产磺onium化合物的方法:其中X代表一个给电子基团;R1和R2各自代表一个烷基、环烷基或类似基团;R3和R4各自代表一个芳香族基或杂芳香族基团;R5和R6各自代表一个烷基、环烷基或类似基团;A−和B−是彼此不同的阴离子。当磺onium化合物用作光酸发生器时,能够产生均匀且优良的抗蚀剂图案。
-
Volatile complexes of some lanthanides and related elements with fluorinated β-diketones and organophosphorus adducts作者:James W. Mitchell、Charles V. BanksDOI:10.1016/s0003-2670(01)95130-x日期:1971.12Abstract The behavior of lanthanide trifluoroacetylacetonates and hexaf luoroacetyl-acetonates of mixed complexes with tri- n -butylphosphate as the adduct has been studied by thermogravimetric methods. Thermal properties of mixed ligand-adduct complexes containing new fluoroorganophosphorus donors have also been examined. Substantial improvements in volatility and thermal stability of chelates of
-
[EN] HEMI-AMINAL ETHERS AND THIOETHERS OF N-ALKENYL CYCLIC COMPOUNDS<br/>[FR] ÉTHERS ET THIOÉTHERS HÉMIAMINAUX DE COMPOSÉS CYCLIQUES N-ALCÉNYLIQUES申请人:ISP INVESTMENTS INC公开号:WO2014116560A1公开(公告)日:2014-07-31Described herein are hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds that may be produced through a reaction comprising: (A) at least one first reactant represented by a structure (I), wherein X is a functionalized or unfunctionalized C1-C5 alkylene group optionally having one or more heteroatoms, and each R1, R2, and R3 is independently selected from the group consisting of hydrogen and functionalized and unfunctionalized alkyl groups optionally having one or more heteroatoms, and (B) at least one second reactant having at least one hydroxyl moiety or thiol moiety. The hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds may comprise a polymerizable moiety, in which case they may be left as-is or used to create homopolymers or non-homopolymers, or they may not comprise a polymerizable moiety. A wide variety of formulations may be created using the hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds, including personal care, oilfield, and construction formulations.
表征谱图
-
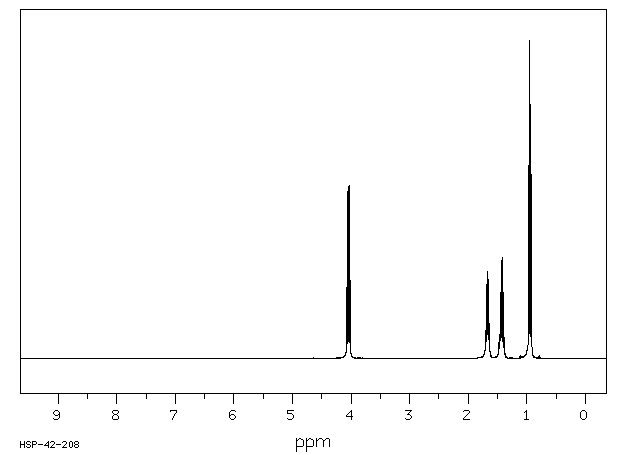
氢谱1HNMR
-
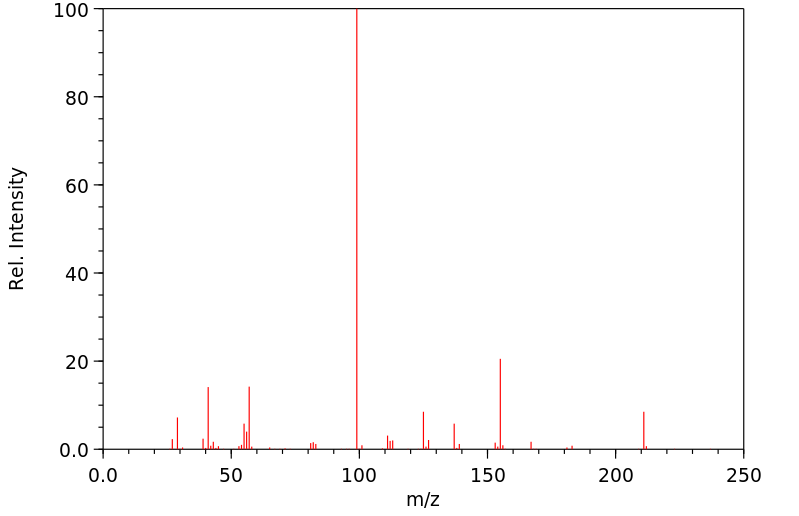
质谱MS
-
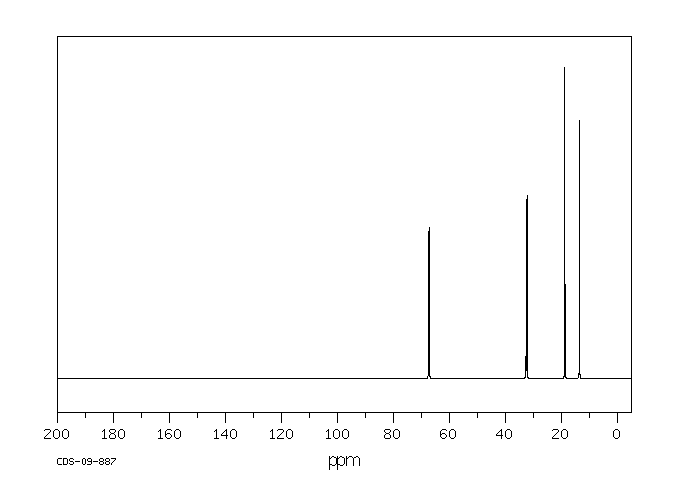
碳谱13CNMR
-
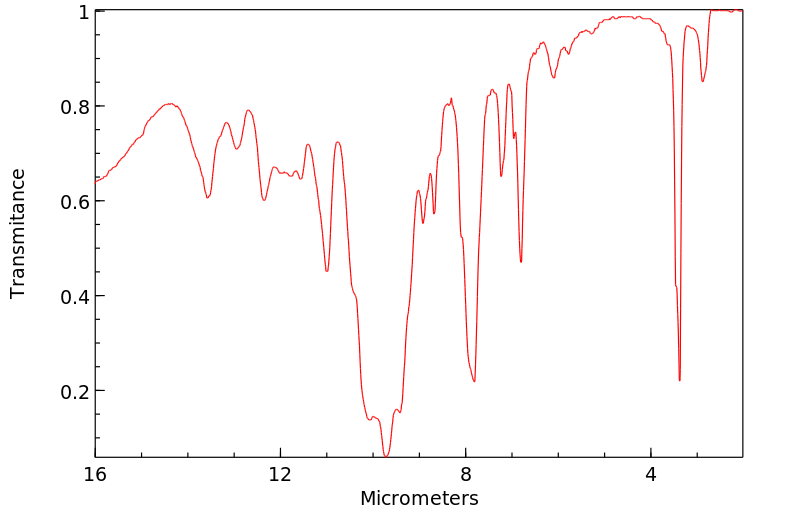
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息