代谢
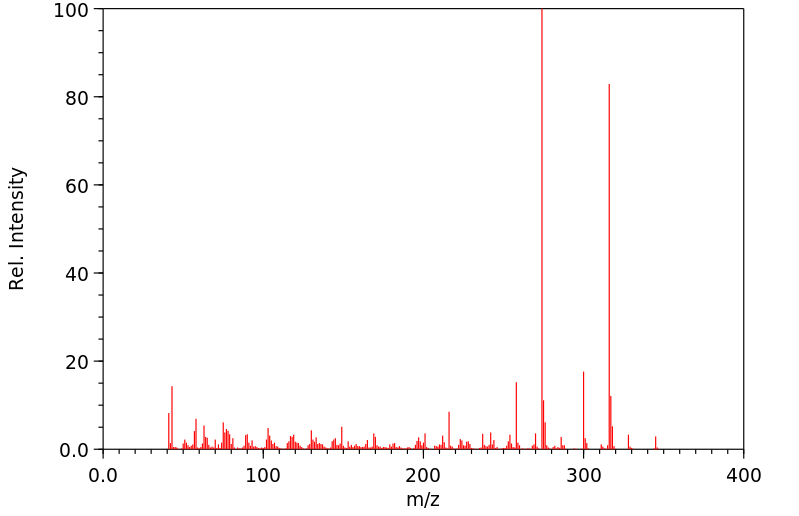
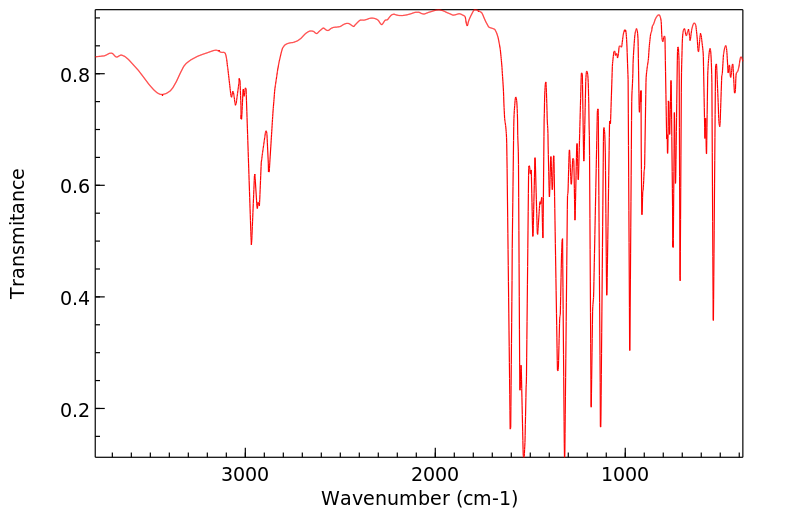
PLANAVIN 以 5 PPM 的浓度通过饲料给了一头插有导管的泌乳期奶牛喂食了 4 天。通过电子亲和力 & 硫火焰光度气相色谱分析,在牛奶、尿液或粪便中没有检测到 PLANAVIN 或可能的含硫代谢物。硫检测器显示,这种除草剂在新鲜瘤胃液中迅速(15分钟)分解,产生了三种含硫的代谢物,但代谢物尚未明确鉴定。
PLANAVIN WAS FED TO A CATHETERIZED LACTATING COW AT 5 PPM IN THE FEED RATION FOR 4 DAYS. PLANAVIN OR POSSIBLE SULFUR-CONTAINING METABOLITES WERE NOT DETECTED IN MILK, URINE OR FECES BY ELECTRON AFFINITY & SULFUR-FLAME PHOTOMETRIC GAS CHROMATOGRAPHIC ANALYSIS. THE SULFUR DETECTOR SHOWED THAT THE HERBICIDE RAPIDLY (15 MIN) DECOMPOSED IN FRESH RUMEN FLUID WITH PRODUCTION OF THREE SULFUR-CONTAINING /SRP: METABOLITES NOT DEFINATELY IDENTIFIED/.
来源:Hazardous Substances Data Bank (HSDB)