4-甲基-4-巯基-2-戊酮 | 19872-52-7
中文名称
4-甲基-4-巯基-2-戊酮
中文别名
4-巯基-4-甲基-2-戊酮,硫甲基戊酮-4,4,2;4-巯基-4-甲基-2-戊酮;硫甲基戊酮-4,4,2;4-甲基-4-巯基-戊-2-酮
英文名称
4-mercapto-4-methylpentan-2-one
英文别名
4-mercapto-4-methyl-2-pentanone;4-methyl-4-sulfanylpentan-2-one;4-sulfanyl-4-methylpentan-2-one
CAS
19872-52-7
化学式
C6H12OS
mdl
MFCD00085208
分子量
132.227
InChiKey
QRNZMFDCKKEPSX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:174℃
-
密度:0.961
-
闪点:54 °C
-
溶解度:可溶于氯仿、甲醇(微量)
-
LogP:1.23
-
物理描述:colourless to pale yellow liquid; roasted meaty aroma
-
折光率:1.431-1.437
-
保留指数:915;915;915;915;915;915;915
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.833
-
拓扑面积:18.1
-
氢给体数:1
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:3
-
安全说明:S26
-
危险类别码:R10,R36/37/38
-
海关编码:2930909090
-
WGK Germany:3
-
包装等级:III
-
危险类别:3
-
危险性防范说明:P261,P305+P351+P338
-
危险品运输编号:1993
-
危险性描述:H225,H319,H335
-
储存条件:存放于惰性气体中,避免与空气接触。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 4-Mercapto-4-methylpentan-2-one
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H226: Flammable liquid and vapour
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: 4-Mercapto-4-methylpentan-2-one
CAS number: 19872-52-7
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
No data
Boiling point:
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C6H12OS
Molecular weight: 132.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
UN Number: UN3336 Class: 3 Packing group: III
Proper shipping name: MERCAPTANS, LIQUID, FLAMMABLE, N.O.S. OR MERCAPTAN MIXTURE, LIQUID, FLAMMABLE
N.O.S. (4-Mercapto-4-methylpentan-2-one)
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 4-Mercapto-4-methylpentan-2-one
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H226: Flammable liquid and vapour
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: 4-Mercapto-4-methylpentan-2-one
CAS number: 19872-52-7
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
No data
Boiling point:
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C6H12OS
Molecular weight: 132.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
UN Number: UN3336 Class: 3 Packing group: III
Proper shipping name: MERCAPTANS, LIQUID, FLAMMABLE, N.O.S. OR MERCAPTAN MIXTURE, LIQUID, FLAMMABLE
N.O.S. (4-Mercapto-4-methylpentan-2-one)
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
反应信息
-
作为反应物:描述:4-甲基-4-巯基-2-戊酮 在 sodium perborate tetrahydrate 、 potassium carbonate 、 溶剂黄146 作用下, 以 甲醇 为溶剂, 反应 24.0h, 生成 4-(allylsulfonyl)-4-methylpentan-2-one参考文献:名称:烷基烯丙基砜的面向多样性的脱磺酰基官能化摘要:通过自由基化学,已开发了具有多种砜类试剂的烷基烯丙基砜的多样性导向型脱磺酰基官能化方法。易于安装的烯丙基磺酰基部分充当C自由基的前体,使用硫基自由基捕获剂将其替换为各种官能团。这种方法的普遍性已由成功的脱磺酰基化炔基化,叠氮化,三氟甲硫基化,亚磺酰基化,三氟甲基硒基化,卤化和氘化证明。该方法与多种官能团兼容。考虑到氘,以高收率掺入氘以高收率获得产物。DOI:10.1002/anie.201903668
-
作为产物:描述:参考文献:名称:A Centrosymmetrical Di-thiolato-bridged Dinuclear Nickel(II) Compound; Bis-µ-S-(7-amino-2,4-dimethyl-5-azahept-4-ene-2-thiolato)dinickel(II) Perchlorate摘要:标题化合物是由 4-巯基-4-甲基戊-2-酮与双(乙烷-1,2-二胺)高氯酸镍(II)反应生成的。 双(乙烷-1,2-二胺)高氯酸镍(II)反应生成的标题化合物,具有一个中心对称的 二核阳离子。每个单态基态镍(II)离子都在四面体 四面体配位。 原子和一分子 7-氨基-2,4-二羟基-2,4-二甲基巯基硫原子的四扭曲方平面配位。 7-氨基-2,4-二甲基-5-氮杂庚-4-烯-2-硫醇酸盐 (adet¯) 分子中的伯胺和亚胺氮原子以及硫醇硫原子与另一分子中的硫原子 分子的硫原子,与两个配体的硫原子形成一个 平面 Ni2S2 桥基 {[Ni2-µ-(adet)2] (ClO4)2、 C16H34C12N4Ni2O8S2、 Mr 662-9,单斜、 P21/c,a 8-267(1)、 b 9-952(1),c 16-234(2)埃,β 103-010(2)°、 R1 0-033 for 2531 反射}。DOI:10.1071/c97005
文献信息
-
Eine einfache Synthese von Cephamdervaten作者:Horst W. Schnabel、Dieter Grimm、Harald JensenDOI:10.1002/jlac.197419740311日期:1974.4.30β-Mercaptocarbonylverbindungen wurden mit 4-Acetoxy-2-azetidinon zu 4-Hydroxycephamderivaten kondensiert und aus diesen nach verschiedenen Methoden Δ3-Cephemderivate und das Δ3-cephem selbst hergestellt.β-mercaptocarbonyl化合物与4-乙酰氧基-2-氮杂环丁酮缩合,得到4- hydroxycepham衍生物和从这些,根据各种方法,Δ 3 -cephem衍生物和Δ 3 -cephem本身制备。
-
BITTER TASTE MODIFIERS INCLUDING SUBSTITUTED 1-BENZYL-3-(1-(ISOXAZOL-4-YLMETHYL)-1H-PYRAZOL-4-YL)IMIDAZOLIDINE-2,4-DIONES AND COMPOSITIONS THEREOF申请人:SENOMYX, INC.公开号:US20160376263A1公开(公告)日:2016-12-29The present invention includes compounds and compositions known to modify the perception of bitter taste, and combinations of said compositions and compounds with additional compositions, compounds, and products. Exemplary compositions comprise one or more of the following: cooling agents; inactive drug ingredients; active pharmaceutical ingredients; food additives or foodstuffs; flavorants, or flavor enhancers; food or beverage products; bitter compounds; sweeteners; bitterants; sour flavorants; salty flavorants; umami flavorants; plant or animal products; compounds known to be used in pet care products; compounds known to be used in personal care products; compounds known to be used in home products; pharmaceutical preparations; topical preparations; cannabis-derived or cannabis-related products; compounds known to be used in oral care products; beverages; scents, perfumes, or odorants; compounds known to be used in consumer products; silicone compounds; abrasives; surfactants; warming agents; smoking articles; fats, oils, or emulsions; and/or probiotic bacteria or supplements.本发明涵盖已知用于改变苦味感知的化合物和组合物,以及所述组合物和化合物与额外的组合物、化合物和产品的组合。示例组合物包括以下一种或多种:冷却剂;无活性药物成分;活性药用成分;食品添加剂或食品;调味剂或调味增强剂;食品或饮料产品;苦味化合物;甜味剂;苦味剂;酸味调味剂;咸味调味剂;鲜味调味剂;植物或动物产品;已知用于宠物护理产品中的化合物;已知用于个人护理产品中的化合物;已知用于家用产品中的化合物;制药制剂;局部制剂;大麻衍生或与大麻相关的产品;已知用于口腔护理产品中的化合物;饮料;香味、香水或除臭剂;已知用于消费品中的化合物;硅化合物;磨料;表面活性剂;发热剂;吸烟物品;脂肪、油脂或乳化剂;和/或益生菌或补充剂。
-
From symmetrical tetrasulfides to trisulfide dioxides <i>via</i> photocatalysis作者:Kai Gong、Yilin Zhou、Xuefeng JiangDOI:10.1039/d1gc03242a日期:——achieved symmetrical tetrasulfides. Stern–Volmer analysis and radical quenching experiments demonstrated the occurrence of a single electron transfer between the photocatalyst and sulfinic acid. Bioactive molecules such as the antihypertensive drug captopril, allicin derivatives, amino acids and terpenes were efficiently and reversibly linked through sulfur–sulfur covalent bonds. Furthermore, flow-setup syntheses
-
CALICHEAMICIN ANTIBODY DRUG CONJUGATES LINKING AN AMIDOACETYL GROUP TO A SUGAR MOIETY ON CALICHEAMICIN申请人:Sorrento Therapeutics, Inc.公开号:US20170281758A1公开(公告)日:2017-10-05There is disclosed a calicheamicin antibody drug conjugate comprising a linking amidoacetyl group covalently bound to a sugar moiety on calicheamicin or linking to sulfur atom on calicheamicin through disulfide bond.
-
Cassis and Green Tea: Spontaneous Release of Natural Aroma Compounds from β‐Alkylthioalkanones作者:Sarah Bättig、Timothy Egger、Olga Gey、Christian G. Bochet、Felix FlachsmannDOI:10.1002/hlca.202100135日期:2021.11are released. Since β-mercaptoketones are potent natural aroma compounds occurring in many fruits, herbs and flowers, the discovery of an enzyme-independent molecular precursor for this class of high-impact molecules is of practical importance. Moreover, the formation of β-diketones and aldehydes by concomitant oxidation at the α-sulfur-position enhances the versatility of this class of aroma precursors
表征谱图
-
氢谱1HNMR
-
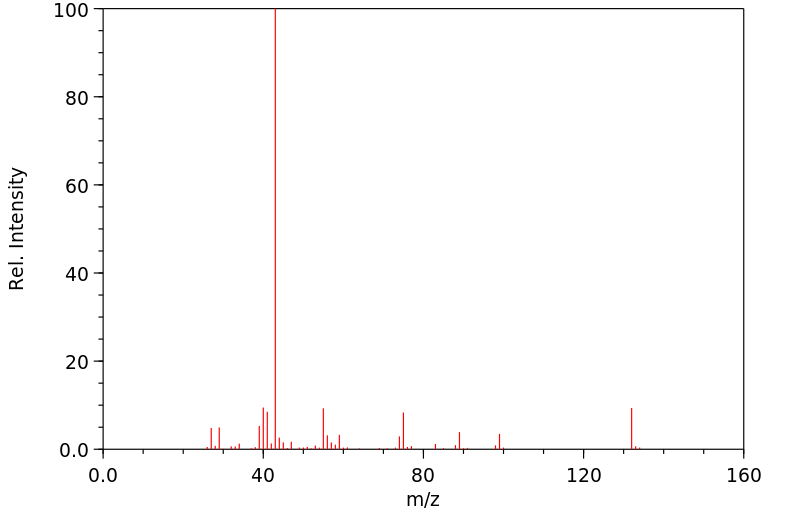
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷