4-苯甲氧基-3-甲氧基苯基乙腈 | 1700-29-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:68-70°C
-
沸点:195°C 1mm
-
密度:1.129±0.06 g/cm3(Predicted)
-
闪点:195°C/1mm
-
稳定性/保质期:
在常温常压下稳定,应避免与强氧化剂或强还原剂接触。
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:19
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.187
-
拓扑面积:42.2
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:6.1
-
危险品标志:Xi
-
安全说明:S22,S36/37
-
危险类别码:R22
-
海关编码:2926909090
-
包装等级:III
-
危险类别:6.1
-
危险品运输编号:3439
-
危险性防范说明:P301+P310
-
危险性描述:H301
-
储存条件:应将储存在冷干燥的条件下,并存放在通风良好、远离加热源及不相容物质的地方,密封保存。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-苄氧基-3-甲氧基苯甲醇 4-benzyloxy-3-methoxybenzyl alcohol 33693-48-0 C15H16O3 244.29 4-苄氧基-3-甲氧基苯甲醛 3-methoxy-4-(phenylmethoxy)benzaldehyde 2426-87-1 C15H14O3 242.274 4-(氯甲基)-2-甲氧基-1-苯基甲氧基苯 1-(benzyloxy)-4-(chloromethyl)-2-methoxybenzene 33688-50-5 C15H15ClO2 262.736 4-(溴甲基)-2-甲氧基-1-苯基甲氧基苯 1-(benzyloxy)-4-(bromomethyl)-2-methoxybenzene 72724-00-6 C15H15BrO2 307.187 4-羟基-3-甲氧基苯乙腈 4-hydroxy-3-methoxyphenylacetonitrile 4468-59-1 C9H9NO2 163.176 —— β-(4-Benzyloxy-3-methoxy-benzyl)-α-thioxopropionsaeure 1700-28-3 C17H16O4S 316.378 4-羟基-3-甲氧基苄醇 4-hydroxymethyl-2-methoxyphenol 498-00-0 C8H10O3 154.166 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-苄氧基-3-甲氧基苯乙胺 2-[4-(benzyloxy)-3-methoxyphenyl]ethylamine 22231-61-4 C16H19NO2 257.332 —— 2-(4-(benzyloxy)-3-methoxyphenyl)-N-methylethanamine 38171-33-4 C17H21NO2 271.359 4-苄氧基-3-甲氧基苯乙酸 4-benzyloxy-3-methoxyphenylacetic acid 29973-91-9 C16H16O4 272.301 —— 2-(4-(benzyloxy)-3-methoxyphenyl)acetyl chloride 54313-35-8 C16H15ClO3 290.746 2-(2-溴-5-甲氧基-4-苯基甲氧基苯基)乙腈 [4-(Benzyloxy)-2-bromo-5-methoxyphenyl]acetonitrile 65341-83-5 C16H14BrNO2 332.197 —— 1-(4-(benzyloxy)-3-methoxyphenyl)cyclobutanecarbonitrile 1260902-74-6 C19H19NO2 293.365 —— 3-Benzyloxy-N-(4-benzyloxy-3-methoxy-phenaethyl)-phenylacetamid 2006-42-0 C31H31NO4 481.591 —— 2-[4-(benzyloxy)-3-methoxyphenyl]-N-[3-(benzyloxy)-4-methoxyphenethyl]acetamide 4672-96-2 C32H33NO5 511.618 N-[4-(苄氧基)-3-甲氧基苯乙基]-2-[3-(苄氧基)-4-甲氧苯基]乙酰胺 (3-benzyloxy-4-methoxy-phenyl)-acetic acid-(4-benzyloxy-3-methoxy-phenethylamide) 1699-40-7 C32H33NO5 511.618
反应信息
-
作为反应物:描述:4-苯甲氧基-3-甲氧基苯基乙腈 在 N-碘代丁二酰亚胺 、 三氟乙酸 作用下, 以 乙腈 为溶剂, 反应 16.0h, 以94%的产率得到4-(benzyloxy)-2-iodo-5-methoxyphenylacetonitrile参考文献:名称:D-环缩合的层状蛋白D的类似物的合成和生物学评估。摘要:合成了具有强细胞毒性的海洋吡咯生物碱lamellarin D的D环收缩类似物,并研究了其对野生型和多药耐药(MDR)癌细胞系的抗增殖作用。发现该化合物在亚微摩尔浓度下抑制肿瘤细胞的生长,并在MDR细胞系中显示出比抗肿瘤药物喜树碱更低的相对抗性,喜树碱对lamellarin D表现出交叉耐药性并且与lamellarin D共享相同的结合位点。DOI:10.1016/j.bmc.2017.02.005
-
作为产物:描述:参考文献:名称:从过量用药生物体液中分离得到的谷氨酰胺亚甲基邻苯二酚代谢产物的鉴定与合成摘要:酶解水解了被谷氨酰胺严重中毒的受害者的尿液样品。TLC,GLC和质谱分析显示母体药物的甲基化邻苯二酚代谢产物。描述了制备2-乙基-2-(3-甲氧基-4-羟基苯基)戊二酰亚胺和2-乙基-2-(3-羟基-4-甲氧基苯基)戊二酰亚胺的两种合成途径。将GLC和质谱数据与从过量的glutethimide受害者体液中分离出来的化合物进行比较,最终确定了一种新的glutethimide的3-甲氧基-4-羟苯基代谢产物。DOI:10.1002/jps.2600680308
文献信息
-
ヘテロシクロアルキル環を含むアミド誘導体申请人:第一三共株式会社公开号:JP2020045306A公开(公告)日:2020-03-26【課題】本発明は、優れたEP300および/またはCREBBPのヒストンアセチルトランスフェラーゼ阻害活性を有する化合物またはその薬理上許容される塩を提供するものである。【解決手段】式(1)で表される化合物またはその薬理上許容される塩。【化1】(ここで、式(1)中の環Q1、環Q2、環Q3、X、およびLは、それぞれ明細書中の定義と同義である。)【選択図】なし本发明提供具有优良的EP300和/或CREBBP的组蛋白乙酰转移酶抑制活性的化合物或其药理上可接受的盐。该化合物或其药理上可接受的盐由式(1)表示。在此,环Q1、环Q2、环Q3、X和L在式(1)中分别与说明书中的定义同义。【选择图】无
-
Biosynthesis. Part 24. Speculative incorporation experiments with 1-benzylisoquinolines and a logical approach via C<sub>6</sub>–C<sub>2</sub>and C<sub>6</sub>–C<sub>3</sub>precursors to the biosynthesis of hasubanonine and protostephanine作者:Alan R. Battersby、Raymond C. F. Jones、Rymantas Kazlauskas、Craig W. Thornber、Somsak Ruchirawat、James StauntonDOI:10.1039/p19810002016日期:——Many possible 1-benzyltetrahydroisoquinolines have been examined as possible advanced precursors of the alkaloids hasubanonine (1) and protostephanine (2) in Stephania japonica plants, but none was incorporated significantly. Administration of various precursor molecules having only one aromatic ring, such as tyrosine, has demonstrated that both alkaloids are derived from two different C6–C2 biogenetic已经研究了许多可能的1-苄基四氢异喹啉类化合物,它们是Stephania japonica植物中生物碱hasubanonine(1)和protostephanine(2)的可能的高级前体,但没有明显掺入。施用仅具有一个芳香环的各种前体分子(例如酪氨酸)已证明这两种生物碱均来自两个不同的C 6 -C 2生物遗传单位。随后无法进一步引入1-苄基四氢异喹啉和双苯乙胺,这表明(a)改性的1-苄基异喹啉或(b)三加氧的C 6 –C 2中间体建筑模块。设计用于检查第一种可能性的前体,例如1-苄基-3,4-二氢异喹啉或1-苄基-1-羧基四氢异喹啉,未合并到(1)和(2)中,而两个3',4',5'-掺入三氧化的2-苯基乙胺。这些发现允许进一步描述对生物碱(1)和(2)的后续前体的需求。
-
A Chemical Probe for Tudor Domain Protein Spindlin1 to Investigate Chromatin Function作者:Vincent Fagan、Catrine Johansson、Carina Gileadi、Octovia Monteiro、James E. Dunford、Reshma Nibhani、Martin Philpott、Jessica Malzahn、Graham Wells、Ruth Faram、Adam P. Cribbs、Nadia Halidi、Fengling Li、Irene Chau、Holger Greschik、Srikannathasan Velupillai、Abdellah Allali-Hassani、James Bennett、Thomas Christott、Charline Giroud、Andrew M. Lewis、Kilian V. M. Huber、Nick Athanasou、Chas Bountra、Manfred Jung、Roland Schüle、Masoud Vedadi、Cheryl Arrowsmith、Yan Xiong、Jian Jin、Oleg Fedorov、Gillian Farnie、Paul E. Brennan、Udo OppermannDOI:10.1021/acs.jmedchem.9b00562日期:2019.10.24basis of a “chromatin or histone code”. Proteins that contain “reader” domains can bind to these modifications and form specific effector complexes, which ultimately mediate chromatin function. The spindlin1 (SPIN1) protein contains three Tudor methyllysine/arginine reader domains and was identified as a putative oncogene and transcriptional coactivator. Here we report a SPIN1 chemical probe inhibitor
-
[EN] Substituted urea-octatydroindols as antagonists of melanin concentrating hormone receptor 1 (MCH1R)<br/>[FR] UREE-OCTAHYDROINDOLES SUBSTITUES UTILISES EN TANT QU'ANTAGONISTES DU RECEPTEUR 1 DE L'HORMONE CONCENTRANT LA MELANINE (MCH1R)申请人:BIOVITRUM AB公开号:WO2005051381A1公开(公告)日:2005-06-09The invention relates to compounds of the general formula (I) wherein R0, R1, R2, R3, R4, R5, R6, R7, R8, R9, Ar, and X are as defined in the description, or a pharmaceutically acceptable salt, hydrates, geometrical isomers, racemates, tautomers, optical isomers, N-oxides and prodrug forms thereof. The compounds may be used for the treatment or prophylaxis of disorders related to the MCH1R receptor and for modulation of appetite. The invention also relates to such use as well as to pharmaceutical formulations comprising a compound of formula (I).该发明涉及通式(I)的化合物,其中R0、R1、R2、R3、R4、R5、R6、R7、R8、R9、Ar和X如描述中所定义,或其药学上可接受的盐、水合物、几何异构体、消旋体、互变异构体、光学异构体、N-氧化物及其前药形式。这些化合物可用于治疗或预防与MCH1R受体相关的疾病,并用于调节食欲。该发明还涉及该用途以及包含通式(I)化合物的药物配方。
-
Structure-Based Design, Docking and Binding Free Energy Calculations of A366 Derivatives as Spindlin1 Inhibitors作者:Chiara Luise、Dina Robaa、Pierre Regenass、David Maurer、Dmytro Ostrovskyi、Ludwig Seifert、Johannes Bacher、Teresa Burgahn、Tobias Wagner、Johannes Seitz、Holger Greschik、Kwang-Su Park、Yan Xiong、Jian Jin、Roland Schüle、Bernhard Breit、Manfred Jung、Wolfgang SipplDOI:10.3390/ijms22115910日期:——
The chromatin reader protein Spindlin1 plays an important role in epigenetic regulation, through which it has been linked to several types of malignant tumors. In the current work, we report on the development of novel analogs of the previously published lead inhibitor A366. In an effort to improve the activity and explore the structure–activity relationship (SAR), a series of 21 derivatives was synthesized, tested in vitro, and investigated by means of molecular modeling tools. Docking studies and molecular dynamics (MD) simulations were performed to analyze and rationalize the structural differences responsible for the Spindlin1 activity. The analysis of MD simulations shed light on the important interactions. Our study highlighted the main structural features that are required for Spindlin1 inhibitory activity, which include a positively charged pyrrolidine moiety embedded into the aromatic cage connected via a propyloxy linker to the 2-aminoindole core. Of the latter, the amidine group anchor the compounds into the pocket through salt bridge interactions with Asp184. Different protocols were tested to identify a fast in silico method that could help to discriminate between active and inactive compounds within the A366 series. Rescoring the docking poses with MM-GBSA calculations was successful in this regard. Because A366 is known to be a G9a inhibitor, the most active developed Spindlin1 inhibitors were also tested over G9a and GLP to verify the selectivity profile of the A366 analogs. This resulted in the discovery of diverse selective compounds, among which 1s and 1t showed Spindlin1 activity in the nanomolar range and selectivity over G9a and GLP. Finally, future design hypotheses were suggested based on our findings.
染色质阅读蛋白Spindlin1在表观遗传调控中扮演重要角色,已与多种恶性肿瘤相关。在当前工作中,我们报道了先前发表的主要抑制剂A366的新类似物的开发。为了提高活性并探索结构-活性关系(SAR),合成了一系列21个衍生物,在体内进行了测试,并通过分子建模工具进行了研究。进行了对接研究和分子动力学(MD)模拟,以分析和理解负责Spindlin1活性的结构差异。MD模拟的分析揭示了重要的相互作用。我们的研究突出显示了用于Spindlin1抑制活性所需的主要结构特征,其中包括嵌入芳香笼中的带正电的吡咯烷基,通过丙氧基连接到2-氨基吲哚核心。在后者中,酰胺基固定化合物到通过与Asp184的盐桥相互作用的口袋中。测试了不同的协议以识别一种快速的计算机辅助方法,可以帮助区分A366系列中的活性和非活性化合物。使用MM-GBSA计算重新评分对接姿势在这方面取得了成功。由于A366被认为是G9a的抑制剂,因此还测试了最活跃的开发的Spindlin1抑制剂对G9a和GLP的选择性配置文件进行验证A366类似物。这导致了多种选择性化合物的发现,其中1s和1t显示出纳摩尔级别的Spindlin1活性,并且对G9a和GLP具有选择性。最后,基于我们的发现提出了未来的设计假设。
表征谱图
-
氢谱1HNMR
-
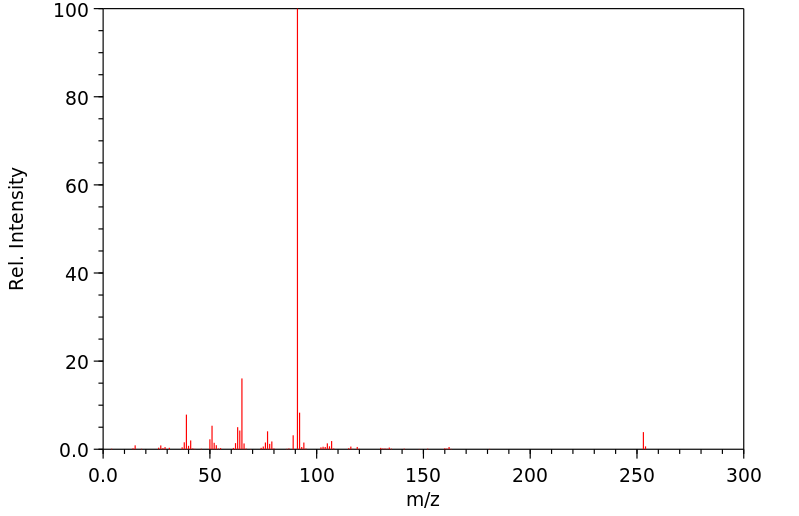
质谱MS
-
碳谱13CNMR
-
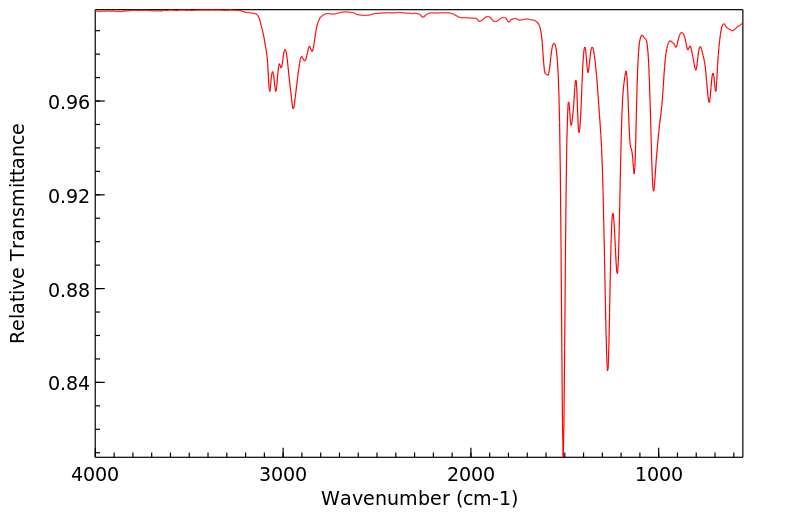
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息