homoterpenylmethylketone | 4436-81-1
中文名称
——
中文别名
——
英文名称
homoterpenylmethylketone
英文别名
5,5-dimethyl-4-(3-oxobutyl)-2(3H)-dihydrofuranone;5,5-dimethyl-4-(3-oxobutyl)dihydro-2(3H)-furanone;Homoterpenyl methyl ketone;5,5-dimethyl-4-(3-oxo-butyl)-dihydro-furan-2-one;(+/-)-3-(α-hydroxy-isopropyl)-6-oxo-heptanoic acid-lactone;(+/-)-3-(α-Hydroxy-isopropyl)-6-oxo-heptansaeure-lacton;2(3H)-Furanone, dihydro-5,5-dimethyl-4-(3-oxobutyl)-;5,5-dimethyl-4-(3-oxobutyl)oxolan-2-one
CAS
4436-81-1
化学式
C10H16O3
mdl
MFCD00022486
分子量
184.235
InChiKey
AWQSAIIDOMEEOD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:278.19°C (rough estimate)
-
密度:1.0683 (rough estimate)
-
保留指数:1558.3
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2932209090
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : 4-HYDROXY-4-METHYL-3-(3-OXOBUTYL)-
VALERIC ACID GAMMA-LACTONE
CAS-No. : 4436-81-1
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Acute toxicity, Oral (Category 4)
Eye irritation (Category 2)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Harmful if swallowed. Irritating to eyes.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Warning
Hazard statement(s)
H302 Harmful if swallowed.
H319 Causes serious eye irritation.
Precautionary statement(s)
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R22 Harmful if swallowed.
R36 Irritating to eyes.
S-phrase(s)
S26 In case of contact with eyes, rinse immediately with plenty of water and
seek medical advice.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C10H16O3
Molecular Weight : 184,24 g/mol
Component Concentration
4-HYDROXY-4-METHYL-3-(3-OXOBUTYL)-VALERIC ACID GAMMA-LACTONE
CAS-No. 4436-81-1 -
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly
investigated.
Indication of immediate medical attention and special treatment needed
no data available
Section 5. FIRE-FIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Precautions for fire-fighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Ensure
adequate ventilation. Avoid breathing dust.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at
the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested and
approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the
standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges. Use
respirators and components tested and approved under appropriate government standards such as
NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting/freezing point no data available
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 0,341
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion Harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes Causes serious eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly
investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed professional
waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent
and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN-Number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for users
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Hueckel; Nerdel, Justus Liebigs Annalen der Chemie, 1937, vol. 528, p. 57,64摘要:DOI:
-
作为产物:描述:参考文献:名称:Cusmano; Linari, Gazzetta Chimica Italiana, 1912, vol. 42 I, p. 6摘要:DOI:
文献信息
-
Development of isoindoline nitroxides for EPR oximetry in viable systems作者:J. Shen、S. Bottle、N. Khan、O. Grinberg、D. Reid、A. Micallef、H. SwartzDOI:10.1007/bf03166117日期:2002.9Nitroxides are widely used as biophysical probes to study molecular motion, intracellular oxygen, pH, transmembrane potential, and cellular redox metabolism, etc. They may be rapidly metabolized to hydroxylamines by cells, which limits their use in viable systems. In this study, we have characterized relevant properties in cells of several isoindoline nitroxides that have been prepared to have different氮氧化物被广泛用作生物物理探针来研究分子运动、细胞内氧、pH、跨膜电位和细胞氧化还原代谢等。它们可能会被细胞迅速代谢为羟胺,这限制了它们在可行系统中的应用。在这项研究中,我们表征了几种异吲哚啉氮氧化物在细胞中的相关特性,这些化合物已制备成具有不同的物理化学特性:1,1,3,3-四甲基异吲哚啉-2-yloxyl (TMIO) 及其类似物 5-carboxy-1, 1,3,3-四甲基异吲哚啉-2-yloxyl (CTMIO), 5-(N,N,N-trimethylammonio)-1,1,3,3-四甲基异吲哚啉-2-yloxyl iodide (QATMIO) 和 2-羟基- 1,1,3,3-四甲基异二氢吲哚盐酸盐 (TMIOH.HCl)。在 CHO 细胞中用 1-oxyl-2、2、6、6-四甲基-4-哌啶酮(Tempone)和3-羧基-2,2,5,5-四甲基-吡咯烷-1-氧基(PCA)。通过
-
Terpenoids Biotransformation in Mammals III: Biotransformation of α-Pinene, β-Pinene, Pinane, 3-Carene, Carane, Myrcene, and p-Cymene in Rabbits作者:T. Ishidax、Y. Asakawa、T. Takemoto、T. ArataniDOI:10.1002/jps.2600700417日期:1981.4The biotransformation of (+)-, (-)-, and (+-)-alpha-pinenes, (-)-beta-pinene (nopinene), (-)-cis-pinane, (+)-3-carene, (-)-cis-carane, myrcene, and p-cymene in rabbits was investigated. The major metabolites were as follows: (-)-trans-verbenol from (+)-, (-)-, and (+/-)-alpha-pinenes; (-)-10-pinanol and (-)-1-p-menthene-7,8-diol from (-)-beta-pinene; (-)-alpha-terpineol and (-)-trans-sobrerol from(+)-,(-)-和(+-)-α-pine烯,(-)-β-pine烯(nopinene),(-)-顺-pin烷,(+)-3-carene,研究了兔体内的(-)-顺式香菜烷,月桂烯和对伞花烃。主要代谢物如下:(+)-,(-)-和(+/-)-α-pine烯的(-)-反式马鞭草酚;(-)-β-pine烯的(-)-10-pin醇和(-)-1-对-薄荷烯-7,8-二醇; (-)-顺式pin烷的(-)-α-松油醇和(-)-反式-细柏油;(-)-m-mentha-4,6-dien-8-ol,3-caren-9-ol,(-)-3-carene-9-羧酸和3-carene-9,10-二羧酸来自(+)-3-carene; (-)-顺式-戊烷中的9,10-二羧酸环烷; 和来自对-异丙基的月桂烯-3(10)-二醇,月桂烯-1,2-二醇,尿萜萜和对-异丙基-9-羧酸。这些新陈代谢包括烯丙基氧化,环氧化,立体选择性的
-
Non-heme iron catalysis in C C, C–H, and CH2 oxidation reactions. Oxidative transformations on terpenoids catalyzed by Fe(bpmen)(OTf)2作者:David Clemente-Tejeda、Alejandro López-Moreno、Francisco A. BermejoDOI:10.1016/j.tet.2013.02.013日期:2013.4The oxidation of terpene olefins with hydrogen peroxide in the presence of the non-hemo catalyst 5a afforded mixtures of epoxides whose composition was dependent upon the oxidation protocol used in each case. With terpenoid enones, the mixtures obtained evolved from clean epoxidation of α-ionone 23 to the clean allylic oxidation of damascone 28 due to the progressive deactivation of the electron density
-
Etudes sur les mati�res v�g�tales volatiles XVII. Sur la synth�se dud,l-?4-car�ne � partir de la pip�rit�none作者:Y. R. Naves、G. PapazianDOI:10.1002/hlca.19420250520日期:1942.8.1L'identité de l'hydrocarbure obtenu par réduction Wolff-Kishner de l'hydrazone de la pipériténone comme d,l-Δ4-carène est confirmée par la caractérisation des produits de son oxydation: l'acide d,l-cis-1,1-diméthyl-2[γ-oxobutyl]-cyclopropane-carboxylique-(3) et les acides d,l-caroniques.
-
The Synthesis of Carvone from α-Terpinyl Acetate作者:Takayuki SugaDOI:10.1246/bcsj.31.569日期:1958.5(1) Oxidation of dl-α-terpineol with tert-butyl chromate yeilded 8-hydroxypiperitone, which has not previously been described, 8-hydroxycarvotanacetone and homoterpenyl methyl ketone. In this case the oxidative fission of the double bond in the ring took place to a remarkable extent.(2) Oxidation of dl-α-terpinyl acetate with tert-butyl chromate gave 8-acetoxypiperitone, 8-acetoxycarvotanacetone, (neither have previously been described) and homoterpenyl methyl ketone. In this oxidation the cleavage of the ethylenic linkage was suppressed, and the yield of 8-acetoxycarvotanacetone was relatively good.Moreover, the effects of the solvent, acetic acid, benzoyl peroxide, air and the reaction temperature, on the yield of 8-acetoxycarvotanacetone wre investigated.(3) 8-acetoxycarvotanacetone was converted into dl-carvone when acetic acid was eliminated by pyrolysis.(4) The synthesis of carvone from α-terpinyl acetate was attiained through the intermeidate of 8-acetoxycarvotanacetone The overall of carvone from α-terpinyl acetate was 28% mole%.
表征谱图
-
氢谱1HNMR
-
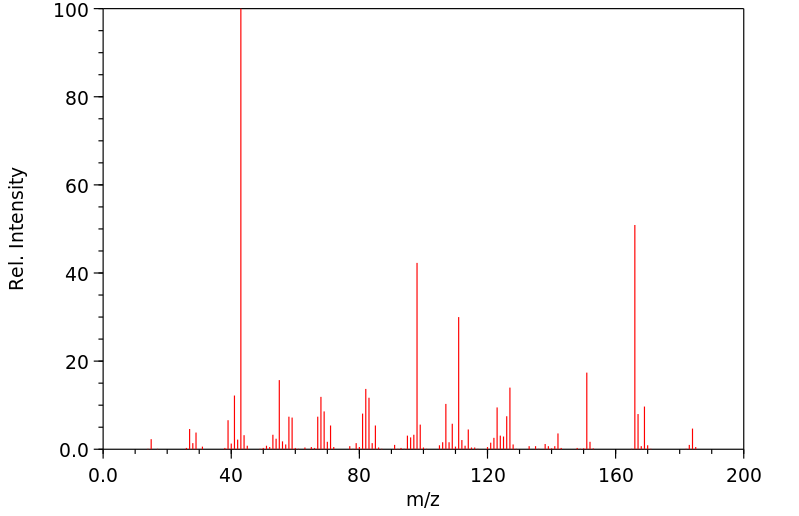
质谱MS
-
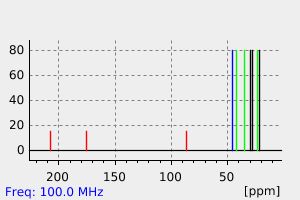
碳谱13CNMR
-
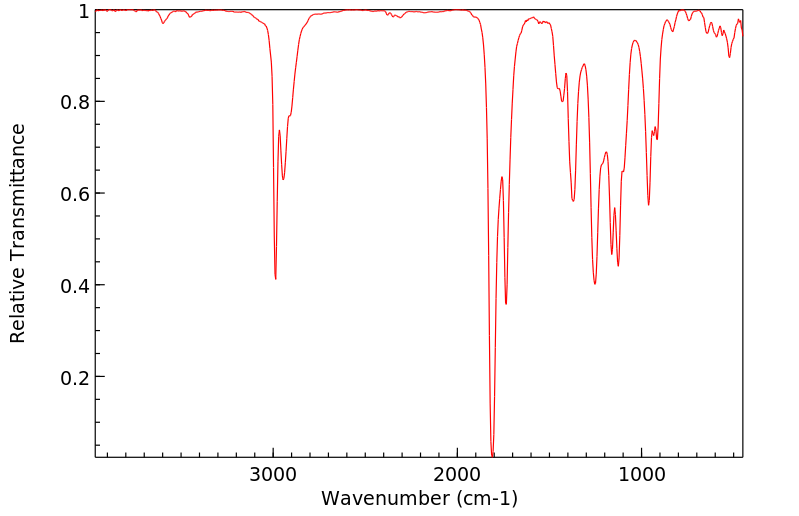
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(+)-(3R)-3-{[叔丁基(二甲基)硅基]氧基}二氢呋喃-2(3H)-酮
龙胆黄碱
龙胆酮胺
高良姜萜内酯
高柠檬酸-gamma-内酯
高普伐他汀内酯二-(叔-丁基二甲基硅烷基)醚
马桑内酯
顺式蒈醛酸内酯
顺式-3,5-二甲基二氢-2H-吡喃-2,6(3H)-二酮
顺式-1,3-环戊烷二甲酸酐
顺式-1,3-环己烷二甲酸酐
阿拉伯酸,2-氨基-2,3,5-三脱氧-3-甲基-,γ-内酯(9CI)
酸,(1S,3R,4R,5R)-3,4-二羟基-7-羰基-6-氧杂二环[3.2.1]辛-1-基2,2,2-三氯乙基酯碳
辛伐他汀4'-甲基醚
辛伐他汀
软木三萜酮3,4-内酯
试剂Menthide
试剂6-Allyl-epsilon-caprolactone
表洛伐他汀
蜂毒
藻酸钠
薇甘菊内酯
葡醛内酯
葡庚糖酸内酯
葡庚糖酸內酯
莫那可林X
莫那可林L
莫那可林J
脱氢抗坏血酸
聚乌拉坦
聚(epsilon-己内酯-delta-戊内酯)
羟基马桑毒内酯
羟基蓍含蓍素
羟基己酸内酯与2,2-二甲基-1,3-丙二醇的聚合物
美伐他汀
绵毛马兜铃内酯
糖质酸-1,4-内酯
穿心莲内酯
科立内脂二醇
硫丹内酯
石蚕苷A
甲酰辛伐他汀
甲瓦龙酸内酯-D4
甲瓦龙酸内酯-D3
甲瓦龙酸内酯-1-13C
甲瓦龙酸内酯-1,2-13C2
甲瓦龙酸内酯
甲基丙烯酸甲瓦龙酸内酯
甲基[(1S,5R,6R)-3-氧代-2-氧杂双环[3.2.1]辛-6-基]乙酸酯
瑞舒伐他汀杂质113