sodium phenylthiosulfonate | 1887-29-2
中文名称
——
中文别名
——
英文名称
sodium phenylthiosulfonate
英文别名
sodium benzenethiosulfonate;sodium benzenesulfonothioate;sodium thiobenzenesulfonate;Sodium;sulfidosulfonylbenzene
CAS
1887-29-2
化学式
C6H5O2S2*Na
mdl
——
分子量
196.226
InChiKey
BZHOWMPPNDKQSQ-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:275 °C (dec.)(lit.)
-
稳定性/保质期:
在常温常压下稳定。
计算性质
-
辛醇/水分配系数(LogP):-2.07
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:80.6
-
氢给体数:0
-
氢受体数:3
安全信息
-
WGK Germany:3
-
海关编码:2930909090
-
储存条件:请将密封存放在干燥、通风的地方。
SDS
| Name: | Benzenethionosulfonic acid sodium salt Material Safety Data Sheet |
| Synonym: | |
| CAS: | 1887-29-2 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1887-29-2 | Benzenethionosulfonic acid, sodium sal | 217-558-8 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1887-29-2: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 275 deg C(decom)
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C6H5NaO2S2
Molecular Weight: 196.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable. Hygroscopic: absorbs moisture or water from the air.
Conditions to Avoid:
Incompatible materials, exposure to moist air or water.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong acids.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, sulfur oxides (SOx), including sulfur oxide and sulfur dioxide.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1887-29-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Benzenethionosulfonic acid, sodium salt - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 1887-29-2: No information available.
Canada
CAS# 1887-29-2 is listed on Canada's NDSL List.
CAS# 1887-29-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1887-29-2 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
反应信息
-
作为反应物:描述:参考文献:名称:S-氟甲基苯硫基磺酸酯的可扩展合成摘要:报道了一种通用且实用的方法,该方法从容易获得的CH 2 FCl,硫和苯亚磺酸钠分两步制备500 g规模的S-氟甲基苯磺酸硫酯。发现该反应对水和温度相当敏感。此外,该反应的主要副产物被鉴定为S-苯基苯磺酸硫代盐。DOI:10.1021/acs.oprd.0c00101
-
作为产物:描述:参考文献:名称:硫代磺酸钠盐:分子和超分子的结构特征以及溶液的辐射分解性能†摘要:通过亚磺酸钠盐与元素硫的直接反应制备了三种硫磺酸钠盐:NaMeS 2 O 2 ·H 2 O,NaPhS 2 O 2和NaMeC 6 H 4 S 2 O 2,这是一种清洁,良性的路线没有副产品。通过X射线晶体学测定苯基(其以水合物形式结晶,NaPhS 2 O 2 ·1.5H 2 O)和对甲苯基化合物的结构。对于对甲苯基衍生物,NaMeC 6 H 4 S 2 O如图2所示,发现了侧基硫原子的出乎意料的配位,这是硫代磺酸盐先前未报道的特征,并且仅在两种较常见的硫代硫酸盐中观察到。假定分子间CH /π相互作用有助于硫配位的驱动力,否则会期望芳环的取向不同。对于NaPhS 2 O 2 ·1.5H 2 O,水配体和硫代磺酸根阴离子各自贡献三个氧原子以形成NaO 6配位球。硫代磺酸盐和水氧与其他钠原子桥连,形成三维层结构,该结构由NaPhS 2 O 2 ·1.5H 2 O片组成,带有亲水性内层,包含钠离子,水配体和-SDOI:10.1039/c1dt10857c
-
作为试剂:描述:N4-benzoyl-3'-O-(methylthiomethyl)-5'-O-(tert-butyldimethylsilyl)-2'-deoxycytidine 、 乙硫醇 在 磺酰氯 、 三乙胺 、 sodium phenylthiosulfonate 作用下, 以 二氯甲烷 、 N,N-二甲基甲酰胺 为溶剂, 反应 3.33h, 以54.4%的产率得到N4-benzoyl-3‘-O-(ethyldithiomethyl)-5‘-O-tert-butyldimethylsilyl-2’-deoxycytidine参考文献:名称:NUCLEOTIDE ANALOGUES摘要:本发明提供了利用包含由甲亚砜二硫醚作为可裂解保护基团的3'-O位置封端的脱氧核苷三磷酸的方法、组合物、混合物和试剂盒,以及可逆地连接到所述脱氧核苷的核碱基的可检测标签。这些化合物为未来的测序技术提供了新的可能性,包括但不限于合成测序。公开号:US20170130051A1
文献信息
-
Copper-Catalyzed Oxidative Trifunctionalization of Olefins: An Access to Functionalized β-Keto Thiosulfones作者:Shuai Huang、Nuligonda Thirupathi、Chen-Ho Tung、Zhenghu XuDOI:10.1021/acs.joc.8b01161日期:2018.8.17Aerobic oxidative trifunctionalization of olefins for the synthesis of functionalized β-keto thiosulfones has been described. The transformation proceeds through molecular oxygen activation under copper catalysis and forms the two new C–S bonds in a single operation using mild conditions. A novel Cu-catalyzed sulfonyl radical addition/oxidation/funtionalization relay mechanism was proposed for the
-
Synthesis of <i>C</i> ‐ and <i>N</i> ‐Substituted 1,5,2,6‐Dithiadiazocanes –Electrophilic‐Nucleophilic Thioamination (ENTA) Reagents作者:Tomas Javorskis、Arminas Jurys、Gintautas Bagdžiūnas、Edvinas OrentasDOI:10.1002/adsc.202100424日期:2021.7A synthetic method is presented for S−N bond formation starting from cheap and affordable materials. We show that (un)substituted N-protected cyclic eight-membered C2-symmetric sulfenamides have been prepared in a few steps using this procedure. The synthetic utility of these ambipolar derivatives was demonstrated in a variety of synthetic transformations affording different S,N-heterocyles of pharmaceutical提出了一种从廉价且负担得起的材料开始形成 S-N 键的合成方法。我们表明,已使用此程序在几个步骤中制备了(未)取代的N保护的环状八元C 2对称次磺酰胺。这些双极性衍生物的合成效用已在各种合成转化中得到证明,这些合成转化可在一个或两个步骤中从简单的起始材料提供具有药物相关性的不同S,N-杂环。
-
Interrupted CuAAC‐Thiolation for the Construction of 1,2,3‐Triazole‐Fused Eight‐Membered Heterocycles from <i>O</i> ‐/ <i>N</i> ‐Propargyl derived Benzyl Thiosulfonates with Organic Azides作者:Raju Jannapu Reddy、Md. Waheed、Arram Haritha Kumari、Gamidi Rama KrishnaDOI:10.1002/adsc.202101256日期:2022.1.18click-sulfenylation of O-/N-propargyl benzyl thiosulfonates with organic azides has been disclosed. The unified CuAAC-thiolation provides a wide range of triazole-fused eight-membered heterocycles in good to high (51–94%) yields under mild reaction conditions. Moreover, a three-component reaction is also achieved involving O-/N-propargyl benzyl thiosulfonates, benzyl bromide, and sodium azide to deliver fused-triazoles
-
Influence of the diamine on the reactivity of thiosulfonato ruthenium complexes with hydrosulfide (HS<sup>−</sup>)作者:Erwan Galardon、Hombeline Daguet、Patrick Deschamps、Pascal Roussel、Alain Tomas、Isabelle ArtaudDOI:10.1039/c2dt31758c日期:——We have recently reported that cationic thiosulfonato ruthenium complexes [(p-cymene)Ru(bipy)(SSO2Ar)]+ (bipy: 2-2â²-bipyridine, Ar: phenyl or p-tolyl) react with thiolates (RSâ, R = alkyl or aryl) by cleavage of the SâSO2 bond and formation of a new SâS bond. In this work, we report that the outcome of the reaction is different if the hydrosulfide anion (R = H) is used, the product obtained being the hydrogen(sulfido) derivative [(p-cymene)Ru(bipy)(SH)]+. The bipy ligand is crucial in this result, and its replacement by ethylenediamine leads to a different product, the trisulfido-bridged dinuclear complex [[(p-cymene)Ru(en)(S)]2S]2+. These two new species have been fully characterized, including by X-ray diffraction studies, and the two different mechanisms leading to their formation are discussed.我们最近报道了阳离子硫代亚磺酸钌配合物 [(p-cymene)Ru(bipy)(SSO2Ar)]+(bipy:2,2'-联吡啶,Ar:苯基或对甲基苯基)与硫醇盐(RS⁻,R = 烷基或芳基)反应,过程为S–SO2键的断裂和新S–S键的形成。在本研究中,我们报告了如果使用氢硫根离子(R = H),反应结果会有所不同,得到的产物为氢(硫化)衍生物 [(p-cymene)Ru(bipy)(SH)]+。bipy配体在这一结果中至关重要,其被乙烯二胺替代则会导致得到不同的产物,即三硫桥联的二核配合物 [[(p-cymene)Ru(en)(S)]2S]2+。这两种新物种已被完全表征,包括X射线衍射研究,并讨论了导致其形成的两种不同机制。
-
Alkynyl Thioethers in Gold-Catalyzed Annulations To Form Oxazoles作者:Raju Jannapu Reddy、Matthew P. Ball-Jones、Paul W. DaviesDOI:10.1002/anie.201706850日期:2017.10.16regioselective, and convergent access to densely functionalized oxazoles is realized in a functional-group tolerant manner using alkynyl thioethers. Sulfur-terminated alkynes provide access to reactivity previously requiring strong donor-substituted alkynes such as ynamides. Sulfur does not act in an analogous donor fashion in this gold-catalyzed reaction, thus leading to complementary regioselective outcomes
表征谱图
-
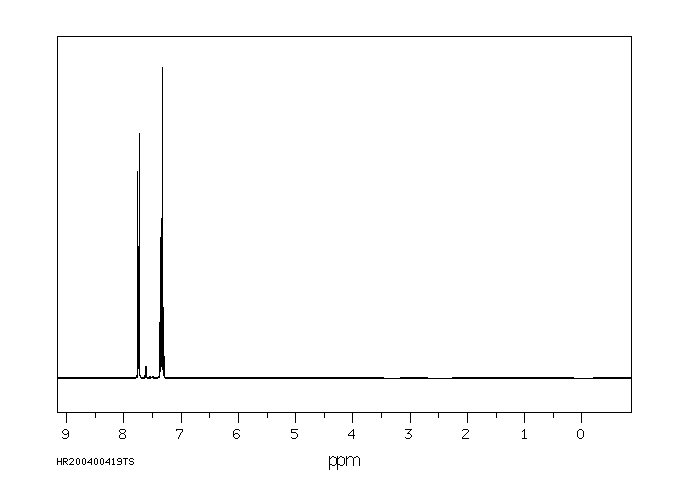
氢谱1HNMR
-
质谱MS
-
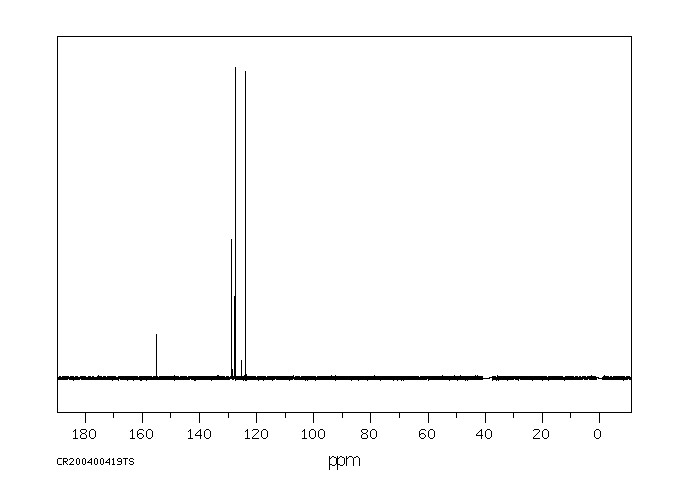
碳谱13CNMR
-
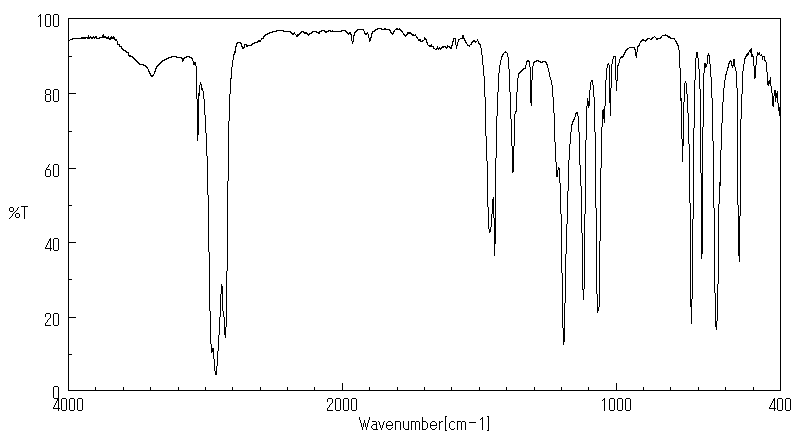
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫