2-chloro-1,2-epoxypropane | 5950-21-0
中文名称
——
中文别名
——
英文名称
2-chloro-1,2-epoxypropane
英文别名
2-chloro-2-methyloxirane;2-Chlor-2-methyl-epoxyaethan
CAS
5950-21-0
化学式
C3H5ClO
mdl
——
分子量
92.5251
InChiKey
RRUZNUBGRZKRNR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:5
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:12.5
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2910900090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Radical reactions of epoxides. Chlorine-atom abstraction from .alpha.- and .beta.-chloro epoxides by the triphenyltin radical摘要:The four isomers of chloroepoxypropane have been prepared, and their relative reactivities with triphenyltin hydride have been determined. The three alpha-chloroepoxypropanes react at a much slower rate than does epichlorohydrin, the only beta-chloro epoxide of the four. The nature of the increased reactivity for the beta-chloro epoxides has been investigated by studying two pair of diastereomeric beta-chloro epoxides, and a single acyclic beta-chloro ether. The results are discussed in terms of the inductive, resonance, and stereoelectronic effects of the epoxide.DOI:10.1021/jo00029a011
-
作为产物:描述:methyloxirane 在 次氯酸叔丁酯 作用下, 以15%的产率得到trans-1-chloro-1,2-epoxypropane参考文献:名称:Radical reactions of epoxides. Chlorine-atom abstraction from .alpha.- and .beta.-chloro epoxides by the triphenyltin radical摘要:The four isomers of chloroepoxypropane have been prepared, and their relative reactivities with triphenyltin hydride have been determined. The three alpha-chloroepoxypropanes react at a much slower rate than does epichlorohydrin, the only beta-chloro epoxide of the four. The nature of the increased reactivity for the beta-chloro epoxides has been investigated by studying two pair of diastereomeric beta-chloro epoxides, and a single acyclic beta-chloro ether. The results are discussed in terms of the inductive, resonance, and stereoelectronic effects of the epoxide.DOI:10.1021/jo00029a011
文献信息
-
Photoinduced reactions of chloroacetone in solid Ar: Identification of CH 2 COClCH 3作者:Nobuaki Tanaka、Yoshitaka Urashima、Hiromasa NishikioriDOI:10.1016/j.cplett.2014.09.053日期:2014.10The UV light-induced reactions of chloroacetone in a cryogenic Ar matrix were investigated using infrared spectroscopy. The photoinduced isomerisations of gauche-chloroacetone to syn-chloroacetone and hypochlorous acid 1-methylethenyl ester were confirmed by comparing the experimental and calculated spectra. In addition, the photolysis products were found to be CH2CO and a cyclopropanone⋯HCl complex
表征谱图
-
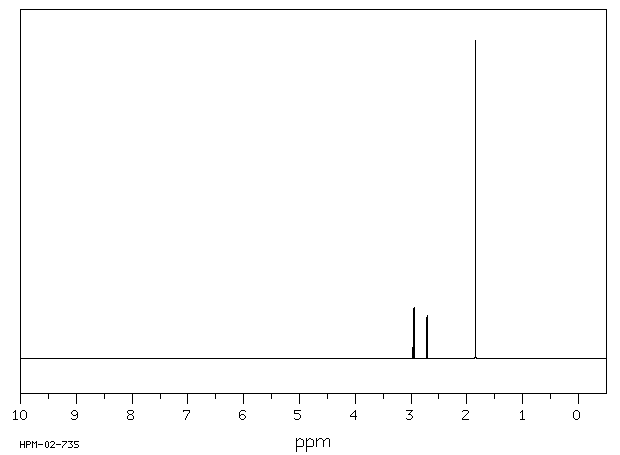
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-氯-1,2-环氧丁烷
顺式-环氧琥珀酸氢钾
顺式-1-环己基-2-乙烯基环氧乙烷
顺-(2S,3S)甲基环氧肉桂酸酯
雌舞毒蛾引诱剂
阿洛司他丁
辛基缩水甘油醚
试剂(3S,6S)-(-)-3,6-Diisopropyl-1,4-dioxane-2,5-dione
表氰醇
螺[环氧乙烷-2,2-三环[3.3.1.1~3,7~]癸烷]
蛇根混合碱
benzene oxide
聚碳酸丙烯酯
聚依他丁
羟基乙醛
缩水甘油基异丁基醚
缩水甘油基十六烷基醚
缩水甘油
硬脂基醇聚氧乙烯聚氧丙烯醚
硅烷,三甲基[(3-甲基噁丙环基)乙炔基]-,顺-
盐酸司维拉姆
甲醛与(氯甲基)环氧乙烷,4,4-(1-甲基乙亚基)双酚和2-甲基苯酚的聚合物
甲醛与(氯甲基)环氧乙烷,4,4'-(1-甲基乙亚基)二[苯酚]和4-(1,1,3,3-四甲基丁基)苯酚的聚合物
甲醇环氧乙烷与壬基酚的聚合物
甲胺聚合物与(氯甲基)环氧乙烷
甲硫代环氧丙烷
甲基环氧氯丙烷
甲基环氧巴豆酸酯
甲基环氧乙烷与环氧乙烷和十六烷基或十八烷基醚的聚合物
甲基环氧乙烷与[(2-丙烯基氧基)甲基]环氧乙烷聚合物
甲基环氧丙醇
甲基环氧丙烷
甲基N-丁-3-烯酰甘氨酸酸酯
甲基7-氧杂双环[4.1.0]庚-2,4-二烯-1-羧酸酯
甲基3-环丙基-2-环氧乙烷羧酸酯
甲基1-氧杂螺[2.5]辛烷-2-羧酸酯
甲基(2S,3R)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3S)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3R)-3-环丙基-2-环氧乙烷羧酸酯
环氧溴丙烷
环氧氯丙烷与双酚A、4-(1,1-二甲乙基)苯酚的聚合物
环氧氯丙烷-d5
环氧氯丙烷-D1
环氧氯丙烷-3,3’-亚氨基二丙胺的聚合物
环氧氯丙烷-2-13C
环氧氯丙烷
环氧氟丙烷
环氧树脂(环氧氯丙烷和二乙二醇)
环氧树脂
环氧柏木烷