cefamandole sodium | 30034-03-8
中文名称
——
中文别名
——
英文名称
cefamandole sodium
英文别名
cefamandole sodium salt;sodium;(2R)-N-[(6R,7R)-2-carboxy-3-[(1-methyltetrazol-5-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-7-yl]-2-hydroxy-2-phenylethanimidate
CAS
30034-03-8
化学式
C18H17N6O5S2*Na
mdl
——
分子量
484.492
InChiKey
OJMNTWPPFNMOCJ-CFOLLTDRSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:>175°C (dec.)
-
溶解度:DMSO(少许)、甲醇(少许)、水(少许)
-
碰撞截面:195.5 Ų [M+Na]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
-
稳定性/保质期:
Moisture Sensitive
计算性质
-
辛醇/水分配系数(LogP):-4.56
-
重原子数:32
-
可旋转键数:7
-
环数:4.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:204
-
氢给体数:2
-
氢受体数:10
安全信息
-
危险品标志:Xn
-
安全说明:S26,S36
-
危险类别码:R36/37/38,R42/43
-
WGK Germany:2
-
RTECS号:XI0389000
-
储存条件:库房应保持低温、通风和干燥。
SDS
制备方法与用途
反应信息
-
作为反应物:参考文献:名称:Orally active esters of cephalosporin antibiotics. 3. Synthesis and biological properties of aminoacyloxymethyl esters of 7-[D-(-)-mandelamido]-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-3-cephem-4-carboxylic acid摘要:The synthesis of six amino acid acyloxymethyl esters of cefamandole (1), a semisynthetic broad-spectrum cephalosporin antibiotic, is described. These esters were examined as potentially useful orally active antibiotic prodrugs. When tested for oral efficacy against Streptococcus pyogenes C203 in mouse protection tests, the esters were not notably more active than lithium cefamandole. Further studies demonstrated that significant blood and urine levels of 1 were not obtained after dosing 2a, 2b, and 2f orally at 17 mg/kg in mice. A study of the stability to chemical hydrolysis and the possible relationship of hydrolysis to the lack of oral absorption of these esters is also presented.DOI:10.1021/jm00192a010
-
作为产物:描述:参考文献:名称:SHIBUYA, CHISEI;MURAKAMI, MASAHIRO;KOBAYASHI, MASATERU;SONE, TAKANORI摘要:DOI:
文献信息
-
1/5水头孢孟多酯钠化合物
-
Novel 3-acyloxymethyl-cephem compounds useful as intermediates for申请人:Takeda Chemical Industries, Ltd.公开号:US04245088A1公开(公告)日:1981-01-13Novel 3-acyloxymethyl-cephem compounds of the formula: ##STR1## wherein R.sup.1 is hydrogen or an acyl group; W is acetonyl, or a group represented by --X--COOH or --X--OH (X is an organic residue) or salts thereof were found to be useful as starting materials for preparing cephalosporins of the formula: ##STR2## wherein R.sup.3 stands for a residue of a nucleophilic compound and R.sup.1 has the same meaning as above.
-
Cephem compounds申请人:Takeda Chemical Industries, Ltd.公开号:US04308381A1公开(公告)日:1981-12-29Novel 3-acyloxymethyl-cephem compounds of the formula: ##STR1## wherein R.sup.1 is hydrogen or an acyl group; W is acetonyl, or a group represented by --X--COOH or --X--OH (X is an organic residue) or salts thereof were found to be useful as starting materials for preparing cephalosporins of the formula: ##STR2## wherein R.sup.3 stands for a residue of a nucleophilic compound and R.sup.1 has the same meaning as above.该文提到了一种公式为:##STR1## 其中R1为氢或酰基;W为乙酰基,或由--X--COOH或--X--OH(X为有机残基)代表的基团或其盐。这些化合物被发现可用作制备公式为:##STR2## 其中R3代表亲核化合物的残基,R1的含义与上述相同的头孢菌素的起始材料。
-
Process for preparing cephalosporins申请人:Takeda Chemical Industries, Ltd.公开号:US04323676A1公开(公告)日:1982-04-06Novel 3-acyloxymethyl-cephem compounds of the formula: ##STR1## wherein R.sup.1 is hydrogen or an acyl group; W is acetonyl, or a group represented by --X--COOH or --X--OH (X is an organic residue) or salts thereof were found to be useful as starting materials for preparing cephalosporins of the formula: ##STR2## wherein R.sup.3 stands for a residue nucleophilic compound and R.sup.1 has the same meaning as above.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
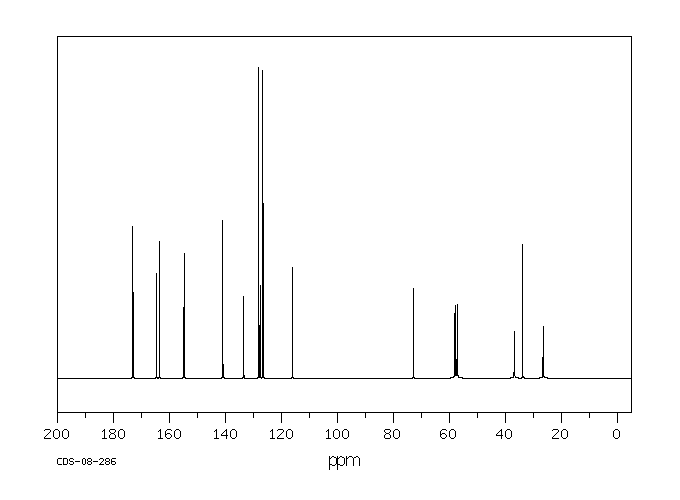
碳谱13CNMR
-
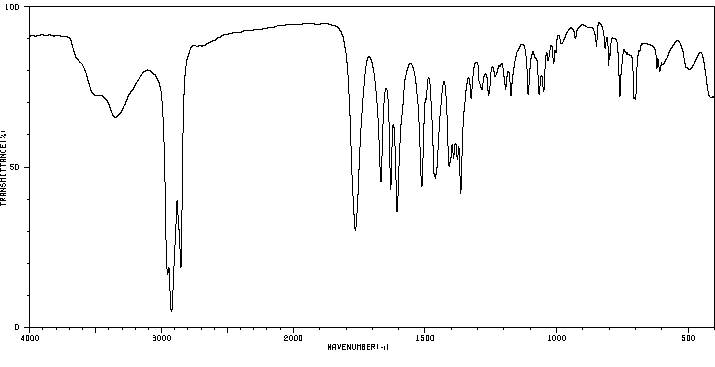
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(6R,7R)-7-苯基乙酰胺基-3-[(Z)-2-(4-甲基噻唑-5-基)乙烯基]-3-头孢唑啉-4-羧酸二苯甲基酯
顺式-4-(2,2-二甲氧基乙基)-3-邻苯二甲酰-2-氮杂环丁酮
顺式-3-氨基-1-(2,4-二甲氧基苄基)-4-甲氧羰基-2-氮杂环丁酮
顺式-1-(对甲苯基)-3-苄氧基-4-(对茴香基)-氮杂环丁烷-2-酮
顺式-1,4-二苯基-3-(甲基苯基氨基)-2-氮杂环丁酮
青霉酰聚赖氨酸
青霉素钾
青霉素钠
青霉素酶液体
青霉素杂质F氢化物
青霉素杂质C
青霉素亚砜酯(GESO)
青霉素V二苄乙二胺
青霉素G衍生物
青霉素G甲酯
青霉素G甲酯
青霉素G-D7
青霉素 V 钠
阿那白滞素
阿莫西林钠
阿莫西林三水合物
阿莫西林
阿立必利D5
阿度西林
铜(2+)酞菁-29,30-二负离子-2-(二甲氨基)乙醇(1:1:1)
钾(2S,5R,6R)-6-[[2-[(E)-3-氯丁-2-烯基]巯基乙酰基]氨基]-3,3-二甲基-7-氧代-4-硫杂-1-氮杂双环[3.2.0]庚烷-2-羧酸酯
钠6-[[3-(2-氯-6-氟苯基)-5-甲基1,2-恶唑-4-羰基]氨基]-3,3-二甲基-7-氧代-4-硫杂-1-氮杂双环[3.2.0]庚烷-2-羧酸盐水合物
钠(6S,7R)-3-(羟基甲基)-7-甲氧基-8-氧代-7-[(2-噻吩基乙酰基)氨基]-5-硫杂-1-氮杂双环[4.2.0]辛-2-烯-2-羧酸酯
钠(6R,7R)-7-[[(2Z)-2-(2-氨基-1,3-噻唑-4-基)-2-甲氧基亚氨基乙酰基]氨基]-8-氧代-3-[(2S)-四氢呋喃-2-基]-5-硫杂-1-氮杂双环[4.2.0]辛-2-烯-2-羧酸酯
钠(2S,5R,6R)-6-[(2-叠氮基-2-苯基乙酰基)氨基]-3,3-二甲基-7-氧代-4-硫杂-1-氮杂双环[3.2.0]庚烷-2-羧酸盐
酞氨西林
赖氨酸氯尼辛
萘夫西林钠
萘夫西林钠
萘夫西林杂质
苯磺酸,2-[(2-羟基-1-萘基)偶氮]-5-甲基-,盐(2:1)钡
苯甘孢霉素亚砜
苯氧乙基青霉素钾
苯并[b]噻吩-3-羧酸,2-[3-氯-2-(4-硝基苯基)-4-羰基-1-吖丁啶基]-4,5,6,7-四氢-,乙基酯
苯唑西林钠
苯唑西林杂质1
舒巴坦杂质19
舒他西林
脱乙酰基戊二酰 7-氨基头孢烷酸
脱乙酰基头孢噻肟
肟莫南
羰苄西林苯酯钠
美罗培南钠盐
美罗培南
美洛培南