cis-diammine(1,1-cyclobutanedicarboxylato) platinum(II) | 41575-94-4
中文名称
——
中文别名
——
英文名称
cis-diammine(1,1-cyclobutanedicarboxylato) platinum(II)
英文别名
1,1-Cyclobutanedicarboxylatodiammineplatinum (cento) (Carboplatin);azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+)
CAS
41575-94-4
化学式
C6H12N2O4Pt
mdl
——
分子量
371.252
InChiKey
VSRXQHXAPYXROS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:228-230°C
-
溶解度:微溶于水,极微溶于丙酮和乙醇(96%)。
-
颜色/状态:White crystals
-
稳定性/保质期:
The manufacturer states that constituted solns are stable for 8 hr at a room temp not exceeding 25 °C. However, other information indicates that the drug may be stable for a much longer time. At a concn of 15 mg/ml in sterile water for injection or at concns of 2 & 0.5 mg/ml in dextrose 5% in water, no decomp occurs in 24 hr at 22-25 °C. Aqueous solns of 10 mg/ml prefilled into plastic syringes exhibited no decomp in 5 days at 4 °C & only a 3% loss in 24 hr at 37 °C.
-
分解:When heated to decomposition it emits toxic fumes of /nitrogen oxides/.
计算性质
-
辛醇/水分配系数(LogP):1.76
-
重原子数:13
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:76.6
-
氢给体数:4
-
氢受体数:6
ADMET
毒理性
轻度和暂时性的血清转氨酶水平升高在多达三分之一的接受卡铂治疗的病人中可以发现。然而,临床上明显的卡铂引起的急性肝损伤极为罕见,这种损伤的特征尚未被很好地定义。此外,卡铂与其他烷化剂联合使用,在高剂量的条件下,用于造血干细胞移植的预处理方案中,可能与窦道阻塞综合症的发生有关,这种综合症可能很严重,并导致急性肝衰竭。窦道阻塞综合症的发病通常在移植后的10到20天内,表现为右上象限疼痛、肝脏压痛、体重增加、水肿和腹水,随后出现黄疸。卡铂在这些窦道阻塞综合症病例中的作用尚未被很好地定义。
卡铂通常与其他抗肿瘤药物联合使用,这些联合使用时发生的不良事件并不总能归因于卡铂。在这方面,有报道称在接受包括卡铂和其他铂配合物(如顺铂和奥沙利铂)的化疗方案后,出现了乙型肝炎的再激活、急性肝坏死、窦道阻塞综合症和严重的高氨血症昏迷(无肝损伤)的个别病例。在大多数这些情况下,很难确定卡铂在造成损伤中的具体作用。
可能性评分:D(可能是临床上明显肝损伤的原因)。
Mild and transient elevations in serum aminotransferase levels are found in up to one-third of patients taking carboplatin. However, clinically apparent acute liver injury from carboplatin is extremely rare and the characteristics of such injury have not been well defined. In addition, carboplatin has been used in combination with other alkylating agents in high doses in conditioning regimens in preparation of hematopoietic cell transplantation which may be associated with instances of sinusoidal obstruction syndrome, which can be severe and lead to acute liver failure. Onset of sinusoidal obstruction syndrome is generally within 10 to 20 days of transplantation and presents with right upper quadrant pain, hepatic tenderness, weight gain, edema and ascites, followed by jaundice. The role of carboplatin in causing these cases of sinusoidal obstruction syndrome has not been well defined.
Carboplatin is usually given in combination with other antineoplastic agents and adverse events that occur with these combinations cannot always be attributed to carboplatin. In this regard, individual case reports of reactivation of hepatitis B, acute hepatic necrosis, sinusoidal obstruction syndrome and severe hyperammonemic coma (without liver injury) have been described after chemotherapeutic regimens that include carboplatin and other platinum coordination complexes such as cisplatin and oxaliplatin. In most of these instances, it is difficult to assign a specific role for carboplatin in causing the injury.
Likelihood score: D (possible cause of clinically apparent liver injury).
来源:LiverTox
毒理性
DILI 注解:较少的药物性肝损伤关注
DILI Annotation:Less-DILI-Concern
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
毒理性
严重性等级:3
Severity Grade:3
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
毒理性
标签部分:警告和预防措施
Label Section:Warnings and precautions
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
安全信息
-
危险品标志:T
-
安全说明:S22,S26,S36/37/39,S45,S53
-
危险类别码:R20/21,R42/43,R46,R61
-
WGK Germany:3
-
海关编码:2843900030
-
危险品运输编号:UN 2811
-
危险标志:GHS07,GHS08
-
危险性描述:H302 + H312 + H332,H317,H334,H340,H360
-
危险性防范说明:P201,P261,P280,P308 + P313
SDS
制备方法与用途
化学性质与物理特性
- 外观和形态:白色絮状疏松粉末或海绵样块状物。
- 溶解性:易溶于水。
- 稳定性:遇光易分解。
-
生物活性:
- 急性毒性(小鼠,腹腔注射)LD50: 150 mg/kg
- 急性毒性(大鼠,静脉注射)LD50: 85 mg/kg
卡铂的制备涉及以下几个步骤:
医学应用- 卵巢癌:有效率为65%
- 睾丸癌
- 小细胞肺癌:有效率为41%
- 头颈部鳞状上皮癌:缓解率为27%
-
常见副作用:
- 骨髓抑制(尤其是血小板减少)是最主要的毒性反应
- 恶心和呕吐(通常延迟出现)
- 脱发
- 常见肝功能异常
-
较少见的副作用:
- 过敏反应
- 口腔炎
- “流感样”综合征
如果患者出现过敏反应,应立即停止用药。轻至中度的过敏反应可通过停药或使用激素治疗来控制症状;而对于严重过敏反应,则需要更积极的支持和对症治疗措施,包括升压药物、呼吸机辅助等。
生产方法总结卡铂主要通过合成顺碘氨铂作为中间体,再与1, 1-环丁烷二羧酸钡在适当条件下进行进一步反应而制备。该过程需严格控制温度及时间以确保产物纯度和收率。
反应信息
-
作为反应物:描述:1,1-环丁基二甲酸 、 cis-diammine(1,1-cyclobutanedicarboxylato) platinum(II) 以93.2的产率得到(DCP)双环铂参考文献:名称:Process for the Preparation of Dicycloplatin摘要:本发明涉及一种在温和反应条件下快速制备二环铂的过程。该过程具有可重复性,易于扩大规模进行工业应用。公开号:US20160297842A1
-
作为产物:描述:1,1-Cyclobutanedicarboxylic Acid Disodium Salt 、 Platinum(4+) nitrate azanide--water (1/2/2/2) 在 1,1-Cyclobutanedicarboxylic Acid Disodium Salt 作用下, 以96.5的产率得到cis-diammine(1,1-cyclobutanedicarboxylato) platinum(II)参考文献:名称:ORGANOMETALLIC COMPOUND摘要:本专利申请涉及一种新型有机金属化合物,其制备过程以及其用途。公开号:US20180055810A1
文献信息
-
一种合成抗肿瘤药物卡铂的新方法申请人:昆明贵研药业有限公司公开号:CN101475600A公开(公告)日:2009-07-08
-
一种抗肿瘤药物卡铂的制备方法申请人:上海金和生物技术有限公司公开号:CN103739631A公开(公告)日:2014-04-23
-
Verfahren zur Herstellung von Carboplatin
-
一种微波催化合成双环铂的方法
表征谱图
-
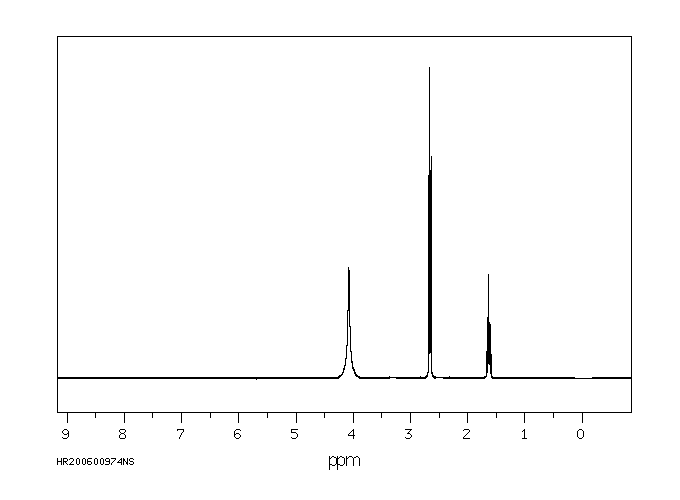
氢谱1HNMR
-
质谱MS
-
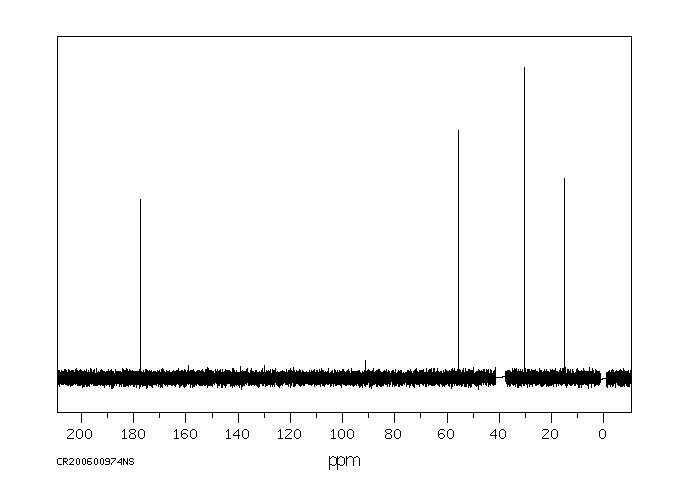
碳谱13CNMR
-
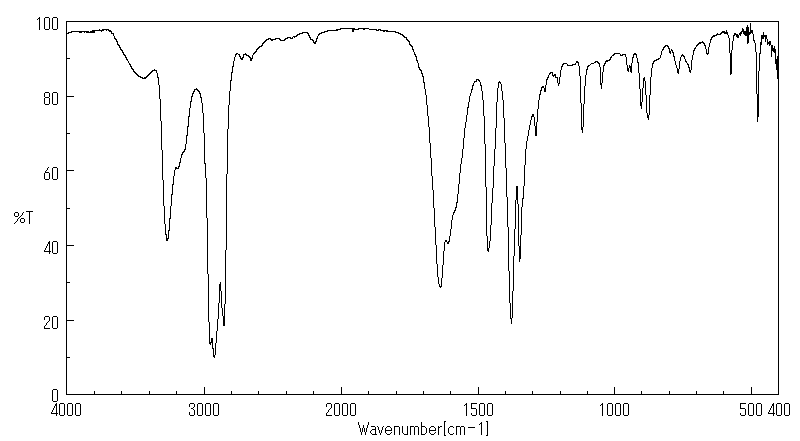
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸