(E)-1-(4-methoxyphenyl)-3-(4-ethoxyphenyl)prop-2-en-1-one | 131887-83-7
分子结构分类
中文名称
——
中文别名
——
英文名称
(E)-1-(4-methoxyphenyl)-3-(4-ethoxyphenyl)prop-2-en-1-one
英文别名
4-ethoxy-4'-methoxychalcone;(E)-3-(4-ethoxyphenyl)-1-(4-methoxyphenyl)prop-2-en-1-one;4-ethoxy-4'-methoxy-trans-chalcone;4-Aethoxy-4'-methoxy-trans-chalkon;3-(4-ethoxyphenyl)-1-(4-methoxyphenyl)-2-propene-1-one;trans-4-Ethoxy-4'-methoxychalcone
CAS
131887-83-7
化学式
C18H18O3
mdl
——
分子量
282.339
InChiKey
OCJRMZSSQYAGCZ-AWNIVKPZSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:21
-
可旋转键数:6
-
环数:2.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对甲氧基苯乙酮 1-(4-methoxyphenyl)ethanone 100-06-1 C9H10O2 150.177
反应信息
-
作为产物:描述:4-乙氧基苯甲醛 、 对甲氧基苯乙酮 在 sodium hydroxide 作用下, 以 乙醇 为溶剂, 生成 (E)-1-(4-methoxyphenyl)-3-(4-ethoxyphenyl)prop-2-en-1-one参考文献:名称:Chalcones and Bis-Chalcones Analogs as DPPH and ABTS Radical Scavengers摘要:背景:许多合成支架物以及天然产物被确定为有效的抗氧化剂。本研究涉及对不同取代、具有药用特性的化合物类别“查尔酮和双查尔酮”进行抗氧化潜力评估。 方法:对一系列合成查尔酮1-13和双查尔酮14-18进行体外自由基清除活性测试。 结果:所有分子1-18在IC50范围内显示出明显的2,2-二苯基-1-苯基-亚硝基肼(DPPH)和2,2'-联苯二(3-乙基苯并噻唑啉-6-磺酸)(ABTS)自由基清除潜力,分别为0.58 ± 0.14 - 1.72 ± 0.03和0.49 ± 0.3 - 1.48 ± 0.06 μM。抗坏血酸(DPPH和ABTS的IC50分别为0.5 ± 0.1和0.46 ± 0.17 μM)被用作标准自由基清除剂。 结论:结构活性关系(SAR)显示了各种基团,包括-SMe和-OMe在清除活性中的积极参与。DOI:10.2174/1570180817999201001155032
文献信息
-
Synthesis and Cytotoxic Evaluation of Alkoxylated Chalcones作者:Xiao-Guang Bai、Chang-Liang Xu、Shuang-Shuang Zhao、Hong-Wei He、Yu-Cheng Wang、Ju-Xian WangDOI:10.3390/molecules191117256日期:——A series of chalcones a1–20 bearing a 4-OMe groups on the A-ring were initially synthesized and their anticancer activities towards HepG2 cells evaluated. Subsequently, a series of chalcones b1–42 bearing methoxy groups at the 2' and 6'-positions of the B-ring were synthesized and their anticancer activities towards five human cancer cell lines (HepG2, HeLa, MCF-7, A549 and SW1990) and two non-tumoral human cell lines evaluated. The results showed that six compounds (b6, b8, b11, b16, b18, b22, b23 and b29) displayed promising activities, with compounds b22 and b29 in particular showing higher levels of activity than etoposide against all five cancer cell lines. Compound b29 showed a promising SI value compared with both HMLE and L02 (2.1–6.5 fold in HMLE and > 33 > 103.1 fold in L02, respectively).
-
Antimicrobial Activity of Xanthohumol and Its Selected Structural Analogues作者:Monika Stompor、Barbara ŻarowskaDOI:10.3390/molecules21050608日期:——which the heterocyclic ring C is closed (flavanones). The strain R. rubra was moderately sensitive to only one compound: 4-hydroxy-4'-methoxychalcone 8. Loss of the hydroxyl group in the B-ring of 4'-methoxychalcones or its replacement by a halogen atom (-Cl, -Br), nitro group (-NO₂), ethoxy group (-OCH₂CH₃), or aliphatic substituent (-CH₃, -CH₂CH₃) resulted in the loss of antimicrobial activity towards这项研究的目的是评估xanthohumol 1(一种在啤酒花中发现的类黄酮化合物)的结构类似物的抗菌活性。应用了使用滤纸盘的琼脂扩散方法。对革兰氏阳性(金黄色葡萄球菌)和革兰氏阴性(大肠埃希氏菌)细菌,真菌(Alternaria sp。)和酵母(Rhodotorula rubra,白色念珠菌)的选定菌株进行的生物学测试显示,至少具有一个羟基的化合物他们所有人都在C-4位置表现出良好的表现。我们的研究表明,金黄色葡萄球菌对查耳酮比对杂环C封闭的异构体(黄酮)更敏感。R. rubra菌株仅对一种化合物中等敏感:4-羟基-4'-甲氧基查耳酮8。4′-甲氧基查尔酮的B环中羟基的丢失或被卤素原子(-Cl,-Br),硝基(-NO 2),乙氧基(-OCH 2 CH 3)或脂族取代基(-CH 3)取代,-CH 2 CH 3)导致对红曲霉酵母和金黄色葡萄球菌的抗菌活性丧失。Xanthohumol 1,柚皮苷
-
Solution-phase parallel synthesis of substituted chalcones and their antiparasitary activity against Giardia lamblia作者:Julio Montes-Avila、Sylvia P. Díaz-Camacho、Josefina Sicairos-Félix、Francisco Delgado-Vargas、I.A. RiveroDOI:10.1016/j.bmc.2009.02.052日期:2009.9A library of 25-membered chalcones was prepared by parallel synthesis. Substituted acetophenones and benzaldehydes were condensed using the Claisen-Schmidt base-catalyzed aldol condensation. Several chalcones showed in vitro antiparasitic activity against Giardia lamblia. The highest activity observed for the IC50 values were 12.72, 15.05 and 15.31 mu g/mL, respectively; these are potential leads for the development of antigiardial compounds. (C) 2009 Elsevier Ltd. All rights reserved.
-
Chalcones and bis-chalcones: As potential α-amylase inhibitors; synthesis, in vitro screening, and molecular modelling studies作者:Adebayo Tajudeen Bale、Khalid Mohammed Khan、Uzma Salar、Sridevi Chigurupati、Tolulope Fasina、Farman Ali、Kanwal、Abdul Wadood、Muhammad Taha、Sitansu Sekhar Nanda、Mehreen Ghufran、Shahnaz PerveenDOI:10.1016/j.bioorg.2018.05.003日期:2018.9Despite of a diverse range of biological activities associated with chalcones and bis-chalcones, they are still neglected by the medicinal chemist for their possible alpha-amylase inhibitory activity. So, the current study is based on the evaluation of this class for the identification of new leads as alpha-amylase inhibitors. For that purpose, a library of substituted chalcones 1-13 and bis-chalcones 14-18 were synthesized and characterized by spectroscopic techniques EI-MS and H-1 NMR. CHN analysis was carried out and found in agreement with the calculated values. All compounds were evaluated for in vitro alpha-amylase inhibitory activity and demonstrated good activities in the range of IC50 = 1.25 +/- 1.05-2.40 +/- 0.09 mu M as compared to the standard acarbose (IC50 = 1.04 +/- 0.3 mu M). Limited structure-activity relationship (SAR) was established by considering the effect of different groups attached to aryl rings on varying inhibitory activity. SMe group in chalcones and OMe group in bis-chalcones were found more influential on the activity than other groups. However, in order to predict the involvement of different groups in the binding interactions with the active site of alpha-amylase enzyme, in silico studies were also conducted.
-
A Study of the Direction of Enolization of p-Methoxy-p'-bromodibenzoylmethane and p-Methoxy-p'-ethoxydibenzoylmethane作者:R. Percy Barnes、Thomas C. Goodwin、Thomas W. CottenDOI:10.1021/ja01204a059日期:1947.12
表征谱图
-
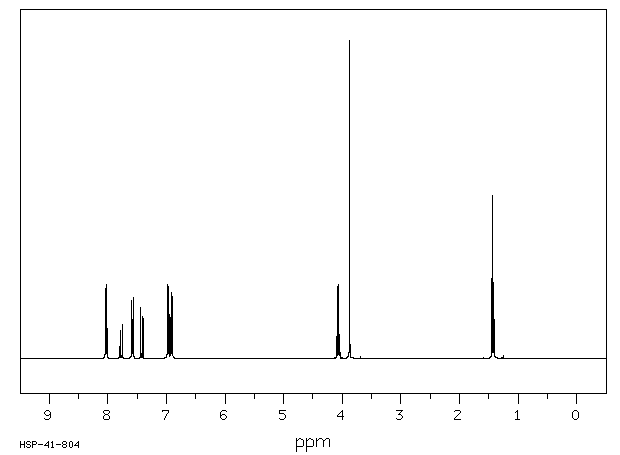
氢谱1HNMR
-
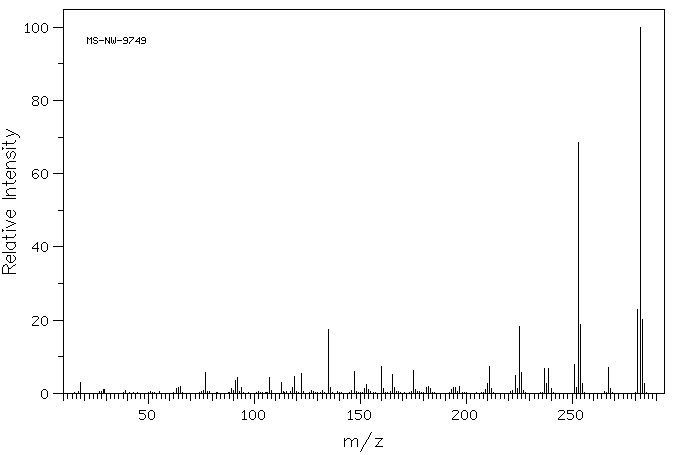
质谱MS
-
碳谱13CNMR
-
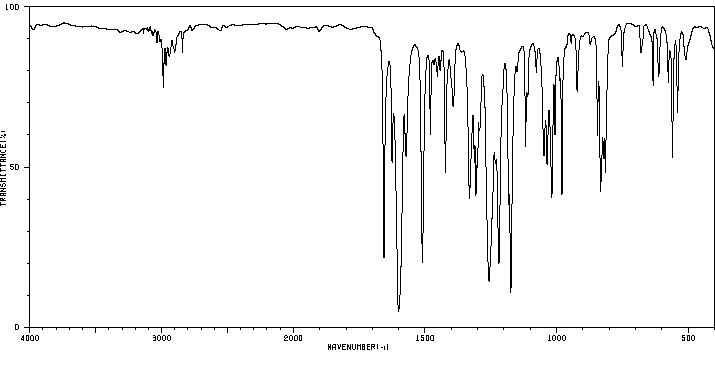
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2Z)-1,3-二苯基-2-丙烯-1-酮,2-丙烯-1-酮,1,3-二苯基-,(2Z)-
龙血素D
龙血素A
龙血素 B
黄色当归醇F
黄色当归醇B
黄腐醇; 黄腐酚
黄腐醇 D; 黄腐酚 D
黄腐酚B
黄腐酚
黄腐酚
黄卡瓦胡椒素 C
高紫柳查尔酮
阿普非农
阿司巴汀
阿伏苯宗
金鸡菊查耳酮
邻肉桂酰苯甲酸
达泊西汀杂质25
豆蔻明
补骨脂色烯查耳酮
补骨脂查耳酮
补骨脂呋喃查耳酮
补骨脂乙素
蜡菊亭; 4,2',4'-三羟基-6'-甲氧基查耳酮
苯酚,4-[3-(2-羟基苯基)-1-苯基丙基]-2-(3-苯基丙基)-
苯磺酰胺,N-[4-[3-(3-羟基苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,N-[3-[3-(4-羟基-3-甲氧苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,4-甲氧基-N,N-二甲基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯化,4,5-二甲氧基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯,4-甲氧基-3-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯甲醇,4-甲氧基-a-[2-(4-甲氧苯基)乙烯基]-
苯甲酸-[4-(3-氧代-3-苯基-丙烯基)-苯胺]
苯甲酸,3-[3-(4-溴苯基)-1-羰基-2-丙烯基]-4-羟基-
苯甲酰(2-羟基苯酰)甲烷
苯甲腈,4-(1-羟基-3-羰基-3-苯基丙基)-
苯基[2-(1-萘基)乙烯基]甲酮
苯基-(三苯基-丙-2-炔基)-醚
苯基-(2-苯基-2,3-二氢-苯并噻唑-2-基)-甲酮
苯亚甲基苯乙酮
苯乙酰腈,a-(1-氨基-2-苯基亚乙基)-
苯丙酸,a-苯甲酰-b-羰基-,苯基(苯基亚甲基)酰肼
苯,1-(2,2-二甲基-3-苯基丙基)-2-甲基-
苏木查耳酮
苄桂哌酯
苄基(4-氯-2-(3-氧代-1,3-二苯基丙基)苯基)氨基甲酸酯
芦荟提取物
腈苯唑
胀果甘草宁C
聚磷酸根皮酚