methyl 4-oxo-2,4-diphenylbutanoate | 5344-60-5
分子结构分类
中文名称
——
中文别名
——
英文名称
methyl 4-oxo-2,4-diphenylbutanoate
英文别名
——
CAS
5344-60-5
化学式
C17H16O3
mdl
——
分子量
268.312
InChiKey
SNCNCZYOCXGYFR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:20
-
可旋转键数:6
-
环数:2.0
-
sp3杂化的碳原子比例:0.18
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2918300090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氧代-2,4-二苯基-丁酸 4-oxo-2,4-diphenylbutanoic acid 4370-96-1 C16H14O3 254.285 3-苯甲酰基-2-苯丙腈 4-oxo-2,4-diphenylbutanenitrile 6268-00-4 C16H13NO 235.285 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-氧代-2,4-二苯基-丁酸 4-oxo-2,4-diphenylbutanoic acid 4370-96-1 C16H14O3 254.285 —— 3-bromo-4-oxo-2,4-diphenyl-butyric acid methyl ester 6267-06-7 C17H15BrO3 347.208 —— 2,4-diphenyl-γ-butyrolactone 21034-29-7 C16H14O2 238.286
反应信息
-
作为反应物:描述:methyl 4-oxo-2,4-diphenylbutanoate 在 selenium(IV) oxide 、 一水合肼 作用下, 以 二乙二醇 为溶剂, 反应 3.62h, 生成 4,6-diphenylpyridazin-3(2H)-one参考文献:名称:固态 4,6-二芳基哒嗪-3(2H)-ONE 的微波辅助合成摘要:微波辅助合成 dihydropyridazin-3(2H)-ones 和随后使用二氧化硒脱氢。DOI:10.1081/scc-100103250
-
作为产物:描述:苯甲酰甲基-二甲基-溴化硫 在 ammonium acetate 、 sodium hydroxide 、 锌 作用下, 以 乙醇 、 水 、 1,2-二氯乙烷 为溶剂, 反应 7.0h, 生成 methyl 4-oxo-2,4-diphenylbutanoate参考文献:名称:可见光促进的多取代烯烃合成,涉及硫叶立德作为碳捕集剂摘要:描述了一种蓝色发光二极管(LED),其促进了重氮芳基乙酸酯与硫酰化物的偶联。该方案具有温和的条件,良好的官能团耐受性以及芳基重氮乙酸酯和硫酰化物的广泛底物范围。在最佳反应条件下,以中等至良好的收率获得了广泛的三取代烯烃,经过两步简单的操作,即可将其进一步转移至其他对生物重要的杂环上。DOI:10.1021/acs.joc.0c02500
文献信息
-
Systematic investigations on the reduction of 4-aryl-4-oxoesters to 1-aryl-1,4-butanediols with methanolic sodium borohydride作者:Subrata Kumar Chaudhuri、Manabendra Saha、Amit Saha、Sanjay BharDOI:10.3762/bjoc.6.94日期:——
4-Aryl-4-oxoesters undergo facile reduction of both the keto and the ester groups with methanolic NaBH4 at room temperature to yield the corresponding 1-aryl-1,4-butanediols whereas 4-alkyl-4-oxoesters furnish the corresponding 1,4-butanolides via selective reduction of the keto moiety. Results of a detailed and systematic investigation of the reaction are described.
-
Photocatalytic Hydromethylation and Hydroalkylation of Olefins Enabled by Titanium Dioxide Mediated Decarboxylation作者:Qilei Zhu、Daniel G. NoceraDOI:10.1021/jacs.0c08688日期:2020.10.21photooxidative redox capacity of the valence band of anatase titanium dioxide (TiO2). Mechanistic studies support a radical-based mechanism involving the photoexcitation of TiO2 with 390-nm light in the presence of acetic acid and other carboxylic acids to generate methyl and alkyl radicals, respectively, without the need for stoichiometric base. This protocol is accepting of a broad scope of alkene
-
Benzothiazolines as radical transfer reagents: hydroalkylation and hydroacylation of alkenes by radical generation under photoirradiation conditions作者:Tatsuhiro Uchikura、Kaworuko Moriyama、Mitsuhiro Toda、Toshiki Mouri、Ignacio Ibáñez、Takahiko AkiyamaDOI:10.1039/c9cc05336k日期:——Novel radical transfer reagents under photoirradiation conditions were developed by the use of benzothiazoline derivatives. These reagents enabled both hydroalkylation and hydroacylation of alkenes under neutral conditions at ambient temperature without any toxic reagents or an excess amount of metals. A mechanistic study was carried out to elucidate the radical process.
-
Photoinduced Deaminative Alkylation for the Synthesis of γ-Ketoesters via Electron Donor–Acceptor Complex Formation作者:Jia-Xin Wang、Wei Ge、Wei-Long Xing、Ming-Chen FuDOI:10.1021/acs.joc.1c02499日期:2021.12.17Visible-light-induced deaminative alkylation of Katritzky salts with silyl enol ethers has been developed. The reaction can proceed efficiently through electron donor–acceptor complex formation, avoiding the use of precious metal complexes or synthetically elaborate organic dyes. A series of functionalized γ-ketoesters was successfully obtained with good functional group tolerance and compatibility under mild
-
New reaction of enamines with aryldiazoacetates catalyzed by transition metal complexes作者:Wei-Jie Zhao、Ming Yan、Dan Huang、Shun-Jun JiDOI:10.1016/j.tet.2005.03.093日期:2005.6The reaction of aryldiazoacetates with enamines catalyzed by copper and rhodium complexes provided γ-keto esters in good yields. A full investigation of the effects of solvents, catalysts, enamines and aryldiazoacetates on the reaction was carried out. Careful analysis of the crude reaction mixture revealed a substituted enamine as the primary product, which was hydrolyzed over silica gel to give a
表征谱图
-
氢谱1HNMR
-
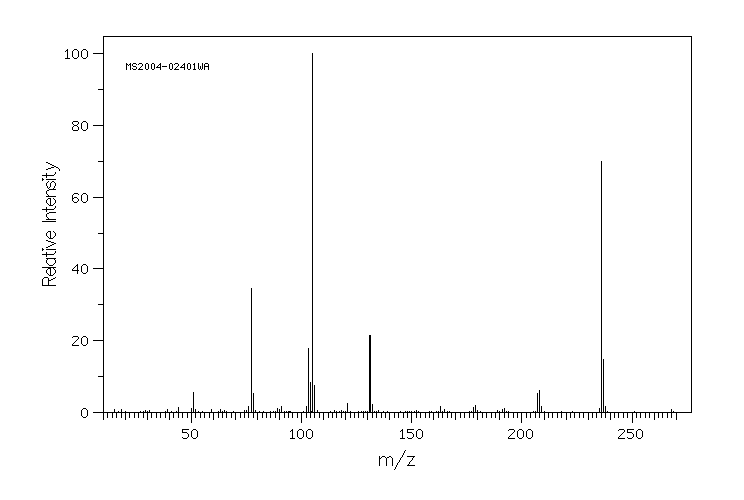
质谱MS
-
碳谱13CNMR
-
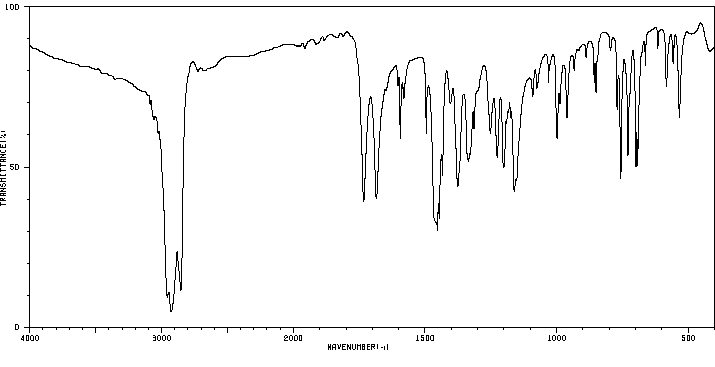
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2Z)-1,3-二苯基-2-丙烯-1-酮,2-丙烯-1-酮,1,3-二苯基-,(2Z)-
龙血素D
龙血素A
龙血素 B
黄色当归醇F
黄色当归醇B
黄腐醇; 黄腐酚
黄腐醇 D; 黄腐酚 D
黄腐酚B
黄腐酚
黄腐酚
黄卡瓦胡椒素 C
高紫柳查尔酮
阿普非农
阿司巴汀
阿伏苯宗
金鸡菊查耳酮
邻肉桂酰苯甲酸
达泊西汀杂质25
豆蔻明
补骨脂色烯查耳酮
补骨脂查耳酮
补骨脂呋喃查耳酮
补骨脂乙素
蜡菊亭; 4,2',4'-三羟基-6'-甲氧基查耳酮
苯酚,4-[3-(2-羟基苯基)-1-苯基丙基]-2-(3-苯基丙基)-
苯磺酰胺,N-[4-[3-(3-羟基苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,N-[3-[3-(4-羟基-3-甲氧苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,4-甲氧基-N,N-二甲基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯化,4,5-二甲氧基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯,4-甲氧基-3-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯甲醇,4-甲氧基-a-[2-(4-甲氧苯基)乙烯基]-
苯甲酸-[4-(3-氧代-3-苯基-丙烯基)-苯胺]
苯甲酸,3-[3-(4-溴苯基)-1-羰基-2-丙烯基]-4-羟基-
苯甲酰(2-羟基苯酰)甲烷
苯甲腈,4-(1-羟基-3-羰基-3-苯基丙基)-
苯基[2-(1-萘基)乙烯基]甲酮
苯基-(三苯基-丙-2-炔基)-醚
苯基-(2-苯基-2,3-二氢-苯并噻唑-2-基)-甲酮
苯亚甲基苯乙酮
苯乙酰腈,a-(1-氨基-2-苯基亚乙基)-
苯丙酸,a-苯甲酰-b-羰基-,苯基(苯基亚甲基)酰肼
苯,1-(2,2-二甲基-3-苯基丙基)-2-甲基-
苏木查耳酮
苄桂哌酯
苄基(4-氯-2-(3-氧代-1,3-二苯基丙基)苯基)氨基甲酸酯
芦荟提取物
腈苯唑
胀果甘草宁C
聚磷酸根皮酚