1,cis-3,cis-5-Trimethylcyclohexane | 1795-27-3
中文名称
——
中文别名
——
英文名称
1,cis-3,cis-5-Trimethylcyclohexane
英文别名
1-cis-3-cis-5-Trimethylcyclohexan
CAS
1795-27-3
化学式
C9H18
mdl
MFCD00070706
分子量
126.242
InChiKey
ODNRTOSCFYDTKF-AYMMMOKOSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-49.69°C
-
沸点:138.55°C
-
密度:0.7670
计算性质
-
辛醇/水分配系数(LogP):3.53
-
重原子数:9.0
-
可旋转键数:0.0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0.0
-
氢给体数:0.0
-
氢受体数:0.0
安全信息
-
海关编码:2902199090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 正丙基环己烷 propylcyclohexane 1678-92-8 C9H18 126.242
反应信息
-
作为反应物:描述:参考文献:名称:Stereoselective dioxirane hydroxylations and the synthesis of tripod boronic acid esters摘要:Methyl(trifluoromethyl)dioxirane (TFDO, 1b), a powerful yet selective oxidant, was employed to achieve in high yield the direct stereoselective hydroxylation at tert-CH of cis,cis-1,3,5-trimethylcyclohexane (4), yielding triol 7 bearing all-axial disposition of the three OH groups. Similarly, TFDO oxidation of 1,3- and of 1,4-dimethylcyclohexane gave the corresponding Z-diols 5 and 6, respectively. Triol 7 was a convenient starting material to synthesize a novel borate-that is, 1-bora-2,8,9-trioxa-3,5,7-trimethyl-adamantane (8)-having a peculiar cage-shaped 'tripod' structure. From triol 7, novel tripod arylboronic Bronsted-assisted Lewis acids (BLA) could be obtained, as exemplified by 10a and 10b. (C) 2007 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2007.03.101
-
作为产物:描述:参考文献:名称:METHODS FOR SELECTIVELY HYDROGENATING SUBSTITUTED ARENES WITH SUPPORTED ORGANOMETALLIC CATALYSTS摘要:提供了一种使用支持的有机金属氢化催化剂选择性地加氢取代芳烃的方法。一个示例方法包括在反应条件下将含有取代芳烃的反应流与氢气接触,在支持的有机金属氢化催化剂存在下,有效地选择性地将取代芳烃加氢成顺式异构体,且选择性高。在这种方法中,支持的有机金属氢化催化剂包括一个具有催化活性的有机金属物种和一个Brønsted酸性硫酸化金属氧化物支撑体。公开号:US20160159709A1
文献信息
-
Polysilane-Immobilized Rh–Pt Bimetallic Nanoparticles as Powerful Arene Hydrogenation Catalysts: Synthesis, Reactions under Batch and Flow Conditions and Reaction Mechanism作者:Hiroyuki Miyamura、Aya Suzuki、Tomohiro Yasukawa、Shu̅ KobayashiDOI:10.1021/jacs.8b06015日期:2018.9.12Hydrogenation of arenes is an important reaction not only for hydrogen storage and transport but also for the synthesis of functional molecules such as pharmaceuticals and biologically active compounds. Here, we describe the development of heterogeneous Rh-Pt bimetallic nanoparticle catalysts for the hydrogenation of arenes with inexpensive polysilane as support. The catalysts could be used in both芳烃的氢化不仅是储氢和运输氢的重要反应,也是合成药物和生物活性化合物等功能性分子的重要反应。在这里,我们描述了非均相 Rh-Pt 双金属纳米颗粒催化剂的开发,用于以廉价的聚硅烷作为载体氢化芳烃。该催化剂可用于间歇和连续流动系统,在温和条件下具有高性能,并显示出广泛的底物通用性。在连续流动系统中,只需将底物和 1 个大气压的 H2 通过装有催化剂的柱子即可获得产物。值得注意的是,在流动系统中观察到比在间歇系统中高得多的催化性能,并且在连续流动条件下表现出极强的耐久性(> 连续运行50天;营业额 >3.4 × 105)。此外,研究了反应机理的细节以及批次和流动中不同动力学的起源,并将获得的知识应用于开发含有两个芳环的化合物的完全选择性芳烃氢化以合成活性药物成分。
-
Copper‐Catalyzed C(sp <sup>3</sup> )−H Amidation: Sterically Driven Primary and Secondary C−H Site‐Selectivity作者:Abolghasem (Gus) Bakhoda、Quan Jiang、Yosra M. Badiei、Jeffery A. Bertke、Thomas R. Cundari、Timothy H. WarrenDOI:10.1002/anie.201810556日期:2019.3.11functionalizing stronger primary and secondary C−H bonds over tertiary and benzylic C−H sites. Herein, we report a Cu catalyst that exhibits a high degree of primary and secondary over tertiary C−H bond selectivity in the amidation of linear and cyclic hydrocarbons with aroyl azides ArC(O)N3. Mechanistic and DFT studies indicate that C−H amidation involves H‐atom abstraction from R‐H substrates by nitrene intermediates无方向的C(sp 3)-H功能化反应通常遵循位点选择性模式,该模式反映了相应的C-H键解离能(BDE)。在存在较强的二级和一级键的情况下,这通常会导致较弱的CHH键的功能化。当代的一个重要挑战是催化剂体系的发展,该催化剂体系能够选择性地在叔和苄基CH位上官能化更强的一级和二级CH键。本文中,我们报道了一种铜催化剂,在线性和环状烃与芳基叠氮化物ArC(O)N 3的酰胺化反应中,叔碳氢键对叔碳氢键的选择性较高。机理和DFT研究表明,C-H酰胺化涉及从由氮宾中间体[铜] R-H的基材H-原子抽象(κ 2 - Ñ,Ö -NC(O)中的Ar),以提供基于碳的基团R 。和铜(II)酰胺中间体[铜II ] -NHC(O)中的Ar,其随后捕获基团R 。形成产品R‐NHC(O)Ar。这些研究揭示了在没有导向基团的情况下实现一级和二级CH酰胺化选择性所需的重要催化剂特征。
-
Absolute kinetics of phenylchlorocarbene CH insertion reactions作者:Robert A. Moss、Shunqi YanDOI:10.1016/s0040-4039(98)02196-0日期:1998.12Absolute rate constants, activation parameters, a kinetic isotope effect, and hybrid density functional theory computational results are presented for various CH insertion reactions of PhCCl.给出了PhCCl各种CH插入反应的绝对速率常数,活化参数,动力学同位素效应和杂化密度泛函理论计算结果。
-
Catalytic hydrogenation of aromatics under biphasic conditions: isolation and structural characterisation of the cluster intermediate [(η6-C6Me6)2(η6-C6H6)Ru3(μ2-H)2(μ2-OH)(μ3-O)]+作者:Matthieu Faure、Ana Tesouro Vallina、Helen Stoeckli-Evans、Georg Süss-FinkDOI:10.1016/s0022-328x(00)00773-7日期:2001.3the hydrogenation of benzene and benzene derivatives to give the corresponding cyclohexanes under biphasic conditions. The catalytic activity of 2 depends markedly on the substrate, an extremely high activity being observed for ethylbenzene. The cationic species present in the catalytic mixture of the ethylbenzene hydrogenation could be isolated as the tetrafluoroborate salt and characterised as the
-
Organoboron compounds作者:M.E. Gurskii、S.V. Baranin、B.M. MikhailovDOI:10.1016/0022-328x(84)80329-0日期:1984.73-Methoxy-7-methyl-3-borabicyclo[3.3.1]nonane (I) and 3-methoxy-7-methoxy-methyl-3-borabicyclo[3.3.1]non-6-ene (X) react with α-halomethyllithium compounds at ⩽ −110°C to yield 3-methoxy-8-methyl-3-borabicyclo[4.3.1]decane and a mixture of 3-methoxy-8-methoxymethyl-3-borabicyclo[4.3.1]dec-7-ene and 3-methoxy-8-methoxymethyl-3-borabicyclo[4.3.1]dec-8-ene. A similar reaction of I or X with α-bromomethyllithium
表征谱图
-
氢谱1HNMR
-
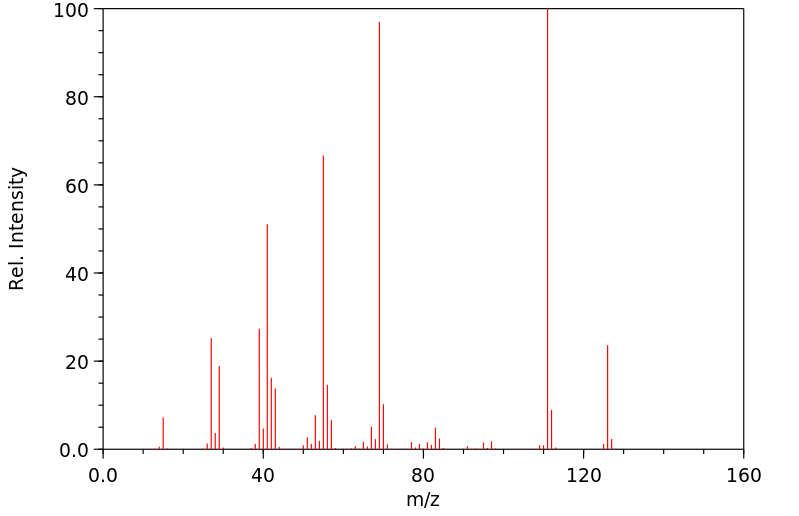
质谱MS
-
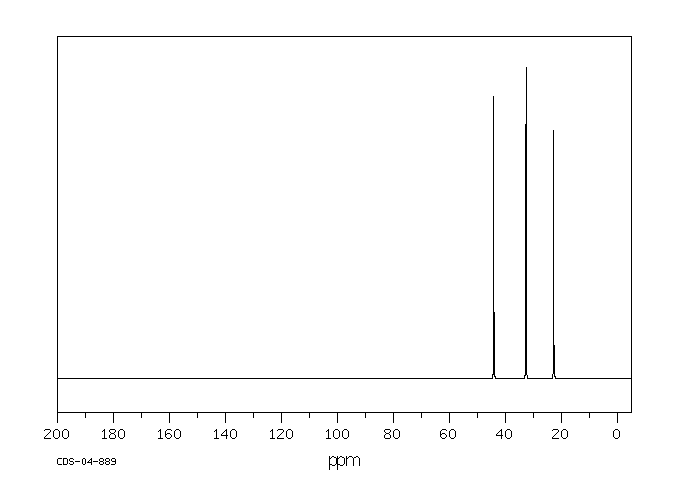
碳谱13CNMR
-
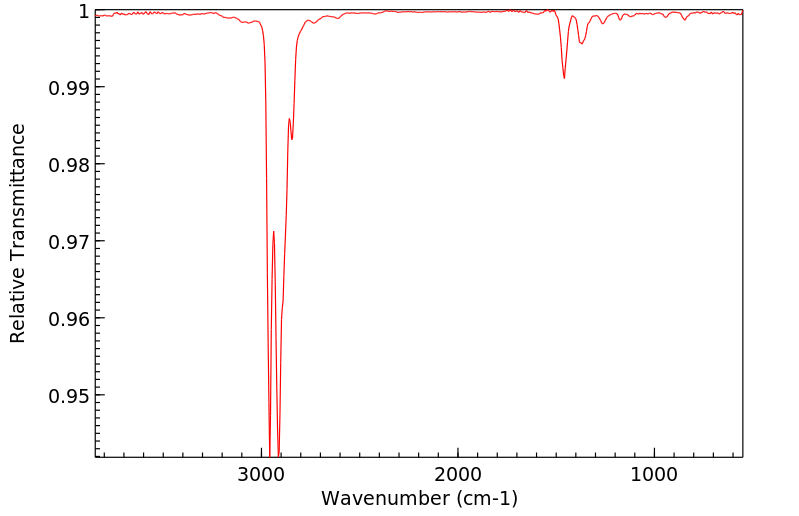
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-1-乙基-3-甲基环己烷
顺式-1-乙基-2-甲基环丙烷
顺式-1,3-二甲基环庚烷
顺式-1,2-二甲基环丙烷
顺式-1,2-二乙基环戊烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式,反式,反式-1,2,4-三甲基环己烷
Copper, ethyl-
辛烷-d18
辛基环戊烷
辛基环丙烷
联苯肼酯
联环戊基
羰基双(环茂二烯基)钛
矿油精
癸烷,2,8-二甲基-
癸烷
decyl radical
癸基环戊烷
異十八烷
甲烷-d3
甲烷-d2
甲烷-d1
甲烷-D4
甲烷-3H
甲烷-13C,d4
甲烷-13C
甲烷
甲基自由基
甲基环辛烷
甲基环癸烷
甲基环戊烷
甲基环己烷-Me-d3
甲基环己烷
甲基环十一烷
甲基环丙烷
甲基环丁烷.
甲基丙烷-2-d
环辛烷-D16
环辛烷
环癸烷
环戊烷-D9
环戊烷-D10
环戊烷-13C1
环戊烷,三(2-辛基十二基)-
环戊烷
环戊基甲基自由基
环戊基环庚烷
环戊基环己烷