octane-2,5-dione | 3214-41-3
中文名称
——
中文别名
——
英文名称
octane-2,5-dione
英文别名
2,5-octanedione;octan-2,5-dione
CAS
3214-41-3
化学式
C8H14O2
mdl
MFCD00043716
分子量
142.198
InChiKey
SPDFSSLYVRCMDZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:105 °C(Press: 15 Torr)
-
密度:0.9635 g/cm3
-
LogP:0.928 (est)
-
保留指数:960;980
-
稳定性/保质期:
存在于主流烟气中。
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:10
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2914190090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 仲辛酮 2-Octanone 111-13-7 C8H16O 128.214
反应信息
-
作为反应物:描述:octane-2,5-dione 在 sodium hydroxide 、 乙醇 作用下, 反应 6.0h, 以70%的产率得到2-Ethyl-3-methyl-cyclopent-2-en-1-on参考文献:名称:Watanabe, Shoji; Fujita, Tsutomu; Suga, Kyoichi, Australian Journal of Chemistry, 1982, vol. 35, # 8, p. 1739 - 1741摘要:DOI:
-
作为产物:描述:diethyl (4-chloro-1-methylthio-3-pentenyl)phosphonate 在 正丁基锂 、 四氯化钛 作用下, 以 甲醇 、 二氯甲烷 、 丙酮 为溶剂, 反应 4.0h, 生成 octane-2,5-dione参考文献:名称:1,4-二酮的便捷合成摘要:摘要 通过2-氯-5-甲硫基-2, 5-己二烯及其与四氯化钛的水解,实现了1,4-二酮的简单和短程合成。DOI:10.1080/00397919108019788
文献信息
-
Neighboring group participation in Lewis acid-promoted [3 + 4] and [3 + 5] annulations. The synthesis of oxabicyclo[3.n.1]alkan-3-ones作者:Gary A. Molander、Kimberly O. CameronDOI:10.1021/ja00056a002日期:1993.22-(alkoxycarbon)-m-oxabicyclo[3.n.1]alkan-3-ones can be constructed by this process in which two new carbon-carbon bonds are generated. Unusually high regioncontrol is observed, and good to excellent stereochemical control can be achieved at virtually every position on the new carbocycles. Intramolecular neighboring group participation is proposed to explain the unusually high selectivities attained in the annulation
-
GLYCOPEPTIDE AND LIPOGLYCOPEPTIDE ANTIBIOTICS WITH IMPROVED SOLUBILITY申请人:Rafai Far Adel公开号:US20120149632A1公开(公告)日:2012-06-14The invention relates to derivatives of glycopeptide and lipoglycopeptide antibiotics possessing an altered ionization state with respect to the parent glycopeptide or lipoglycopeptide antibiotic, and having the ability to be regenerated as the parent glycopeptide or lipoglycopeptide antibiotic under physiological conditions. These compounds are useful as antibiotics for the prevention and/or the treatment of bacterial infections.这项发明涉及具有与母体糖肽或脂质糖肽抗生素相比具有改变的电离状态的糖肽和脂质糖肽抗生素衍生物,并且具有在生理条件下能够再生为母体糖肽或脂质糖肽抗生素的能力。这些化合物可用作预防和/或治疗细菌感染的抗生素。
-
Un anion β-acyle masque dans les reactions d'acylation: Le derive lithie du dioxolanne du levulate de trimethylsilyle作者:Jean-Louis Moreau、René CouffignalDOI:10.1016/0022-328x(85)87462-3日期:1985.10An organolithium reagent derived from trimethylsilyl-4,4-ethylenedioxypentanoate reacts with mixed carboxylic-carbonic anhydrides as a homoenolate anion equivalent. Several monoethylene acetals of 1,4-diketones and the corresponding diketones are synthesized by this way.
-
Synthesis of 2,5-Disubstituted Pyrrolidine Alkaloids <i>via</i> A One-Pot Cascade Using Transaminase and Reductive Aminase Biocatalysts作者:Bruna Z. Costa、James L. Galman、Iustina Slabu、Scott P. France、Anita J. Marsaioli、Nicholas J. TurnerDOI:10.1002/cctc.201801166日期:2018.10.23A multi‐enzymatic cascade process involving transaminases (TAs) and reductive aminases (RedAms) to produce enantiomerically pure 2,5‐disubstituted pyrrolidine alkaloids from their respective 1,4‐diketones is reported. Several TAs were screened and the best results for diketone monoamination were obtained with an R‐selective TA from Mycobacterium chlorophenicum and with an S‐selective TA from Bacillus
-
Process for the preparation of ketones申请人:Bayer Aktiengesellschaft公开号:US04035395A1公开(公告)日:1977-07-12The invention concerns a new process for the preparation of ketones; according to this process ketones are prepared from aldehydes and unsaturated compounds in the presence of bases using quaternary ammonium salts as catalysts.
表征谱图
-
氢谱1HNMR
-
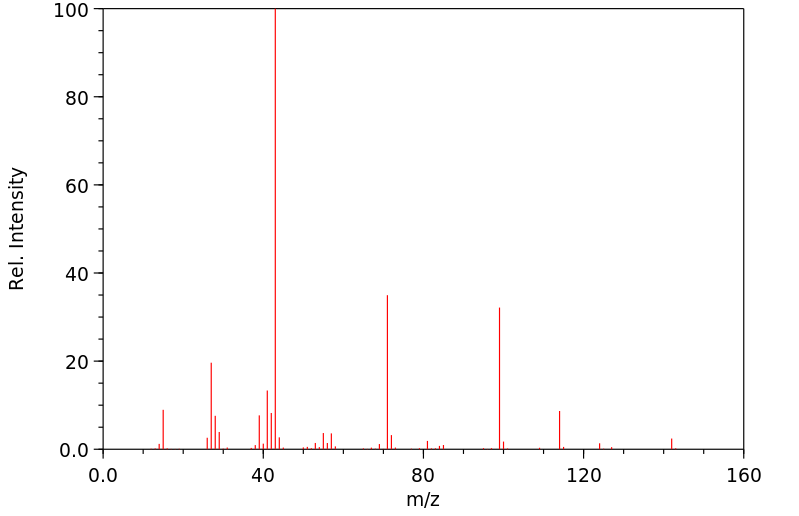
质谱MS
-
碳谱13CNMR
-
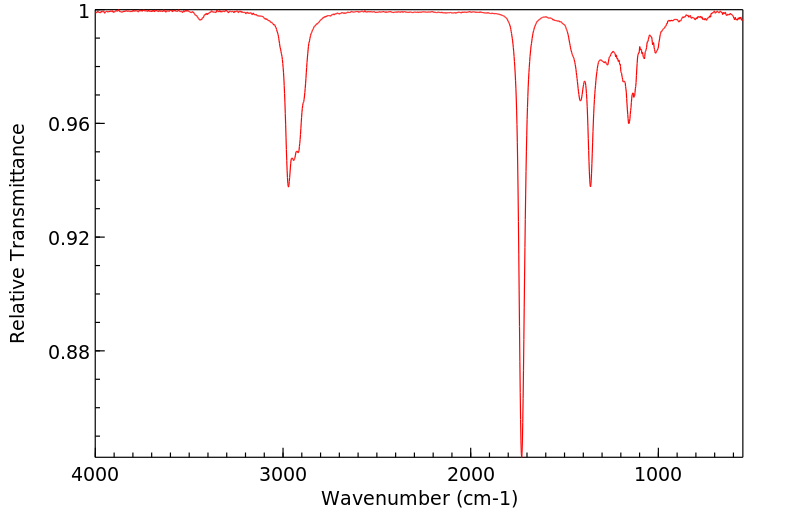
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷