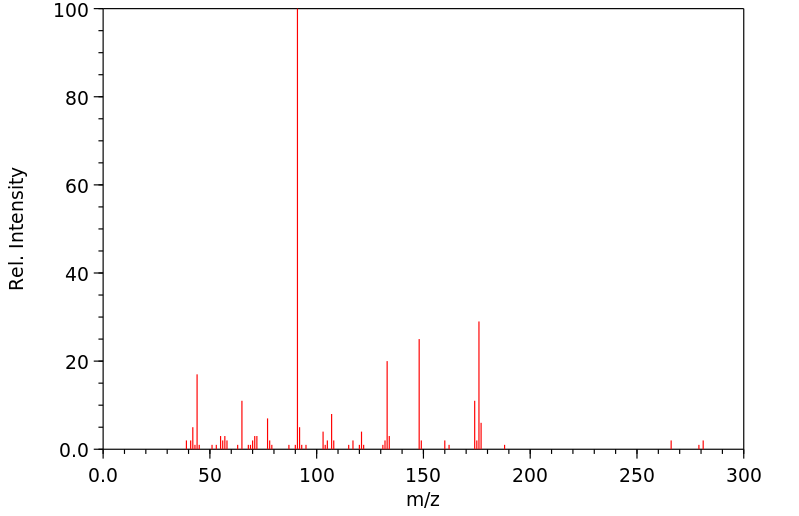
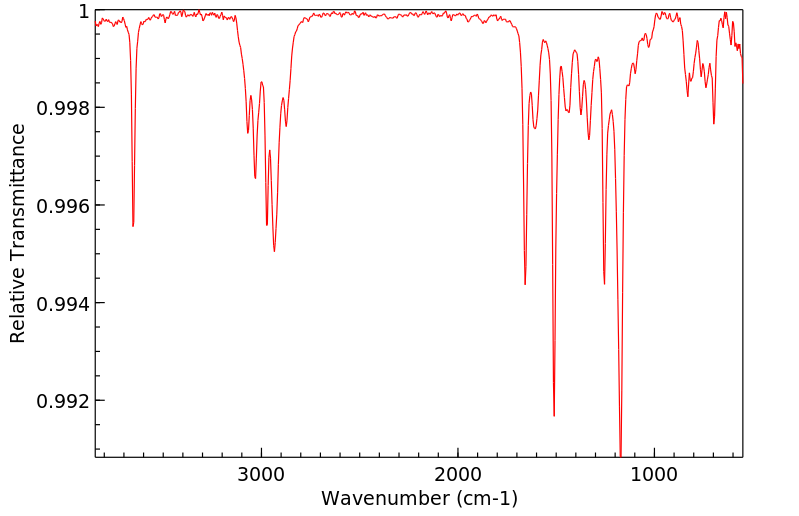
毒理性
含有
吩噻嗪结构的
抗组胺药的效果可以通过尼利德林增强,尼利德林通过从受体部位和组织中取代
吩噻嗪,以及通过尼利德林的脑血管扩张作用来实现。尽管缺乏临床文献支持,
吩噻嗪类药物的活性可能会因同时使用尼利德林而增强。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
在大鼠中预先使用尼利定处理可保护其免受
氰化钠急性毒性的影响。
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/SRP:/ 基本治疗:建立专利气道(如有需要,使用口咽或鼻咽气道)。如有必要,进行吸痰。密切观察呼吸不足的迹象,如有需要,进行辅助通气。通过非循环呼吸面罩以10至15升/分钟的速度给予
氧气。监测肺
水肿,并在必要时进行治疗……。监测休克,并在必要时进行治疗……。预测癫痫发作,并在必要时进行治疗……。对于眼睛污染,立即用
水冲洗眼睛。在运输过程中,用0.9%的生理盐
水(NS)持续冲洗每只眼睛……。不要使用催吐剂。对于摄入,如果患者能够吞咽、有强烈的咳嗽反射且不流口
水,则用
水冲洗口腔,并给予5毫升/千克,最多200毫升的
水进行稀释……。在去污后,用干性无菌敷料覆盖皮肤烧伤……。/毒物A和B/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/SRP:/ 高级治疗:对于昏迷、严重肺
水肿或严重呼吸困难的病人,考虑进行口咽或鼻咽气管插管以控制气道。使用气囊面罩装置的正压通气技术可能有益。考虑使用药物治疗肺
水肿...。对于严重的支气管痉挛,考虑给予β激动剂,如
沙丁胺醇...。监测心率和必要时治疗心律失常...。开始静脉输注5%
葡萄糖水(D5W)/SRP: "保持开放",最低流量/。如果出现低血容量的迹象,使用0.9%盐
水(NS)或
乳酸林格液。对于伴有低血容量迹象的低血压,谨慎给予液体。注意液体过载的迹象...。使用
地西泮或
劳拉西泮治疗癫痫...。使用
丙美卡因氢
氯化物协助眼部冲洗...。/Poisons A and B/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/其他毒性信息/ 通过β-
肾上腺素受体刺激以及可能的直接作用,导致骨骼动脉和小动脉的血管扩张。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
容易从胃肠道吸收。
来源:DrugBank
吸收、分配和排泄
作用持续时间:2小时。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
达到峰值效果的时间:30分钟。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
起效时间:10分钟。
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
容易从胃肠道吸收。
来源:Hazardous Substances Data Bank (HSDB)