6-庚烯-3-醇 | 19781-77-2
中文名称
6-庚烯-3-醇
中文别名
5-醇基-1-庚烯;四烯丙基溴化铵
英文名称
hept-6-en-3-ol
英文别名
6-hepten-3-ol
CAS
19781-77-2
化学式
C7H14O
mdl
——
分子量
114.188
InChiKey
XFXWEAWJVWCOBF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:26°C (estimate)
-
沸点:178.73°C (estimate)
-
密度:0.8596 (estimate)
-
LogP:1.639 (est)
-
稳定性/保质期:
如果按照规则使用和储存,则不会分解。
避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:8
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.71
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:3
-
危险类别码:R10,R36/37/38
-
危险品运输编号:UN 1987
-
海关编码:2905290000
-
包装等级:III
-
危险类别:3
-
储存条件:在密封的贮藏器中保存,并将其置于阴凉、干燥处。
SDS
反应信息
-
作为反应物:描述:参考文献:名称:高锰酸十六烷基三甲基铵的取代基定向氧化环化:合成γ-和δ-内酯的一般方法摘要:用高锰酸十六烷基三甲基铵处理伯,仲或叔γ-和δ-羟基烯烃,可通过氧化环化获得良好的γ-和δ-内酯收率,且损失一个碳。DOI:10.1016/s0040-4039(00)84916-3
-
作为产物:参考文献:名称:用于制备磺内酯衍生物的闭环复分解 (RCM) 和金属催化的环丙烷化摘要:应用Grubbs催化剂2代作为催化剂的闭环复分解(RCM)制备了一系列高产率的新型不饱和磺内酯。使用二乙基锌/二碘甲烷或 Zn-Cu/二碘甲烷通过 Simmon-smith 环丙烷化将烯丙基磺内酯的环丙烷化应用了许多尝试,但在每种情况下均未形成相应的环状加合物。不饱和磺内酯与重氮乙酸乙酯的新型钯或优选铑催化的环丙烷化反应是通过过渡金属催化转移 CH-CO2Et 单元实现的。在低温(0-20℃)下,通过在6小时内向磺内酯和乙酸钯(II)或乙酸铑(II)二聚体的混合物中分批加入重氮乙酸乙酯来进行反应。在每种情况下都获得了所需的环丙烷化产物,DOI:10.24820/ark.5550190.p010.749
文献信息
-
Iron(II) and Copper(I) Control the Total Regioselectivity in the Hydrobromination of Alkenes作者:Daniel A. Cruz、Victoria Sinka、Pedro de Armas、Hugo Sebastian Steingruber、Israel Fernández、Víctor S. Martín、Pedro O. Miranda、Juan I. PadrónDOI:10.1021/acs.orglett.1c02186日期:2021.8.6A new method that allows the complete control of the regioselectivity of the hydrobromination reaction of alkenes is described. Herein, we report a radical procedure with TMSBr and oxygen as common reagents, where the formation of the anti-Markovnikov product occurs in the presence of parts per million amounts of the Cu(I) species and the formation of the Markovnikov product occurs in the presence
-
Nickel-Catalyzed Sonogashira Reactions of Non-activated Secondary Alkyl Bromides and Iodides作者:Jun Yi、Xi Lu、Yan-Yan Sun、Bin Xiao、Lei LiuDOI:10.1002/anie.201307069日期:2013.11.18A nicked reaction: The title reaction of terminal alkynes with non‐activated secondary alkyl iodides and bromides was accomplished for the first time. This reaction provides a new and practical approach for the synthesis of substituted alkynes (see scheme; cod=cyclo‐1,5‐octadiene).
-
Intramolecular hydroalkoxylation in Brønsted acidic ionic liquids and its application to the synthesis of (±)-centrolobine作者:Yunkyung Jeong、Do-Young Kim、Yunsil Choi、Jae-Sang RyuDOI:10.1039/c0ob00701c日期:——The SO3H-tethered imidazolium and triazolium salts, nonvolatile and recyclable Brønsted acidic ionic liquids, efficiently mediate intramolecular hydroalkoxylations of alkenyl alcohols. They have been successfully employed in the synthesis of (±)-centrolobine.
-
Copper-Catalyzed Oxysulfenylation of Enolates with Sodium Sulfinates: A Strategy To Construct Sulfenylated Cyclic Ethers作者:Yinglan Gao、Yang Gao、Xiaodong Tang、Jianwen Peng、Miao Hu、Wanqing Wu、Huanfeng JiangDOI:10.1021/acs.orglett.6b00272日期:2016.3.4A new copper-catalyzed oxysulfenylation reaction of enolates with sodium sulfinates has been disclosed. A series of sulfenylated heterocycles including four- and seven-membered cyclic ether were obtained in mild to good yields. This reaction is proposed to go through a radical process, and the sulfur radical (RS•) may be a reactive species.
-
Rhodium-Phosphoramidite Catalyzed Alkene Hydroacylation: Mechanism and Octaketide Natural Product Synthesis作者:Max von Delius、Christine M. Le、Vy M. DongDOI:10.1021/ja305593y日期:2012.9.12heterogeneous base are key for high catalytic activity and linear regioselectivity. This protocol was applied in the atom- and step-economical synthesis of eight biologically active octaketide natural products, including anticancer drug candidate cytosporone B. Mechanistic studies provide insight on parameters affecting decarbonylation, a side reaction that limits the turnover number for catalytic hydroacylation我们描述了一种方法,该方法允许水杨醛衍生物以低至 2 mol% 的催化剂负载量与各种未活化的烯烃偶联。手性亚磷酰胺配体和多相碱的精确化学计量是高催化活性和线性区域选择性的关键。该协议应用于八种生物活性八酮天然产物的原子和步骤经济合成,包括抗癌药物候选细胞孢菌素 B。 机理研究提供了对影响脱羰的参数的见解,这是一种限制催化加氢酰化转换数的副反应。氘标记研究表明,支化氢化物插入是完全可逆的,而线性氢化物插入在很大程度上是不可逆的并且限制了转换。我们建议配体 (R(a),R, R)-SIPHOS-PE 通过降低线性选择性途径中还原消除的障碍,有效抑制脱羰,并有助于限制转换的插入。这些因素共同实现了高反应性和区域选择性。
表征谱图
-
氢谱1HNMR
-
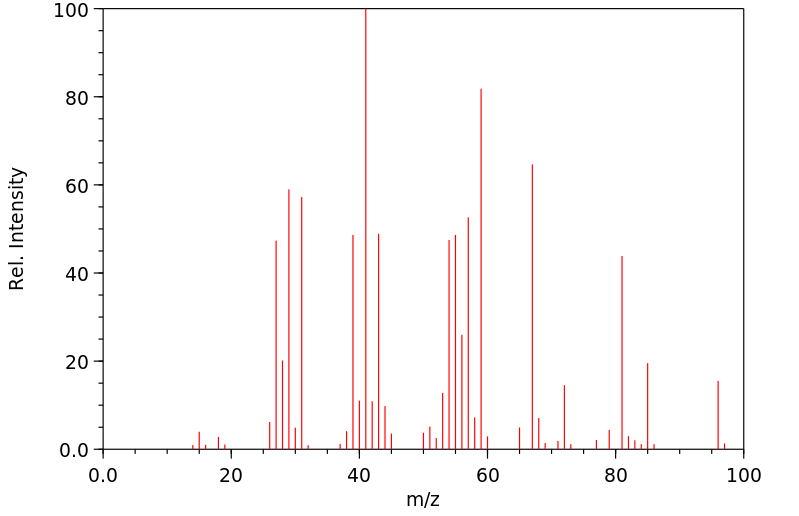
质谱MS
-
碳谱13CNMR
-
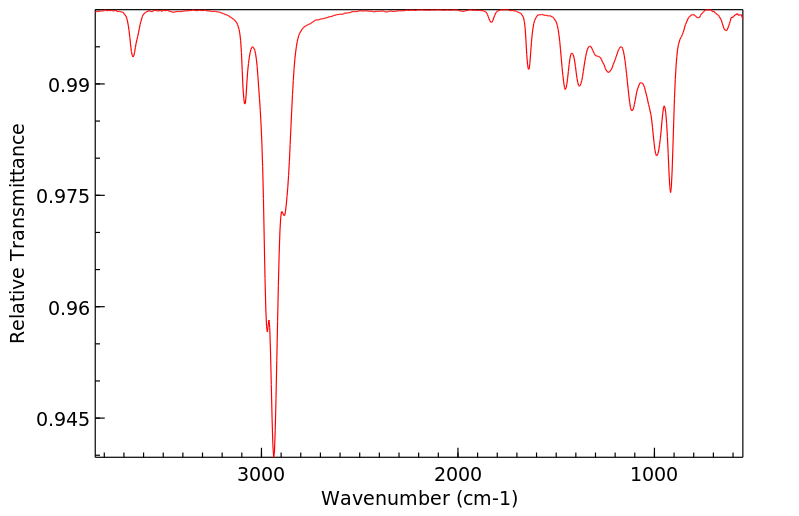
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷