N,N-二乙基甘氨酸甲酯 | 30280-35-4
中文名称
N,N-二乙基甘氨酸甲酯
中文别名
——
英文名称
N,N-diethylglycine methyl ester
英文别名
methyl diethylamino-acetate;Diethylaminoessigsaeuremethylester;methyl 2-(diethylamino)acetate
CAS
30280-35-4
化学式
C7H15NO2
mdl
MFCD00059368
分子量
145.202
InChiKey
YLZLHVWLTRZOJH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:86 °C / 57mmHg
-
密度:0.93
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,也未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):0.9
-
重原子数:10
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.857
-
拓扑面积:29.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:3
-
海关编码:2922499990
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥处。同时,确保工作环境具有良好的通风或排气设施。
SDS
N,N-Diethylglycine Methyl Ester Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: N,N-Diethylglycine Methyl Ester
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 3
Flammable liquids
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Flammable liquid and vapour
Precautionary statements:
[Prevention] Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Wear protective gloves/eye protection/face protection.
[Response] IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
[Storage] Store in a well-ventilated place. Keep cool.
Dispose of contents/container through a waste management company authorized by
[Disposal]
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: N,N-Diethylglycine Methyl Ester
Percent: >98.0%(GC)(T)
CAS Number: 30280-35-4
Chemical Formula: C7H15NO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
N,N-Diethylglycine Methyl Ester
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in an explosion-poof refregerator.
Store under inert gas.
Protect from moisture.
Store away from incompatible materials such as oxidizing agents.
Heat-sensitive, Moisture-sensitive
Comply with laws.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
N,N-Diethylglycine Methyl Ester
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Clear
Form:
Colour: Colorless - Yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 86°C/7.6kPa
No data available
Flash point:
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.93
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Conditions to avoid: Spark, Open flame, Static discharge
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
N,N-Diethylglycine Methyl Ester
Section 12. ECOLOGICAL INFORMATION
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 3: Flammable liquid.
1993
UN-No:
Proper shipping name: Flammable liquid, n.o.s.
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: N,N-Diethylglycine Methyl Ester
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 3
Flammable liquids
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Flammable liquid and vapour
Precautionary statements:
[Prevention] Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Wear protective gloves/eye protection/face protection.
[Response] IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
[Storage] Store in a well-ventilated place. Keep cool.
Dispose of contents/container through a waste management company authorized by
[Disposal]
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: N,N-Diethylglycine Methyl Ester
Percent: >98.0%(GC)(T)
CAS Number: 30280-35-4
Chemical Formula: C7H15NO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
N,N-Diethylglycine Methyl Ester
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in an explosion-poof refregerator.
Store under inert gas.
Protect from moisture.
Store away from incompatible materials such as oxidizing agents.
Heat-sensitive, Moisture-sensitive
Comply with laws.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
N,N-Diethylglycine Methyl Ester
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Clear
Form:
Colour: Colorless - Yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 86°C/7.6kPa
No data available
Flash point:
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.93
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Conditions to avoid: Spark, Open flame, Static discharge
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
N,N-Diethylglycine Methyl Ester
Section 12. ECOLOGICAL INFORMATION
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 3: Flammable liquid.
1993
UN-No:
Proper shipping name: Flammable liquid, n.o.s.
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Indazole compounds, compositions thereof and methods of treatment therewith摘要:这项发明通常涉及使用吲唑化合物来治疗或预防与蛋白激酶相关的疾病,包括酪氨酸激酶,诸如增殖性疾病、炎症性疾病、异常血管生成及其相关疾病、动脉硬化、黄斑变性、糖尿病、肥胖、疼痛等。这些方法包括向有需要的患者施用有效量的吲唑化合物,以抑制、调节或控制酪氨酸激酶信号转导。本文中提供了一种新型的吲唑化合物或其药用可接受的盐。公开号:US20050009876A1
-
作为产物:参考文献:名称:Willstaetter, Chemische Berichte, 1902, vol. 35, p. 602摘要:DOI:
-
作为试剂:描述:2-氨基-2-乙基丁酸甲酯 在 sodium hydroxide 、 N,N-二乙基甘氨酸甲酯 、 1-(3-二甲基氨基丙基)-3-乙基碳二亚胺 作用下, 以 1,4-二氧六环 、 水 、 乙腈 为溶剂, 反应 480.0h, 生成 methyl trifluoroacetyl-diethylglycyl-diethylglycyl-diethylglycinate参考文献:名称:Helicalversus Planar Conformation of Homooligopeptides Prepared from Diethylglycine (=2-Amino-2-ethylbutanoic Acid)摘要:Homooligopeptides containing alpha,alpha-diethylgycine (= 2-amino-2-ethylbutanoic acid), were synthesized by conventional solution methods. An ethyl or methyl ester was used as protecting group at the C-terminus and a trifluoroacetyl group as protecting group at the N-terminus of the peptides. The conformations of such tri-, penta-, and hexapeptides in the solid stare were studied using X-ray crystallographic analysis, and were shown to be a bent planar CS-conformation in the case of tripeptide 8a, and a 3(10)-helical structure in the case of pentapeptide 10 and hexapeptide 11. IR and H-1-NMR spectra revealed that the dominant conformation of hexapeptide 11 in CDCl3 solution was not the 3(10)-helical structure shown in the solid state, but a fully planar C5 structure.DOI:10.1002/(sici)1522-2675(19990407)82:4<494::aid-hlca494>3.0.co;2-a
文献信息
-
Efficient oxidative N -dealkylative addition of trialkylamines to dimethyl acetylenedicarboxylate using BrCCl 3 as the terminal oxidant作者:Fangnan Liu、Zhiguo Zhang、Zongbi Bao、Huabin Xing、Yiwen Yang、Qilong RenDOI:10.1016/j.tetlet.2017.05.073日期:2017.7Herein we present a continuous improvement of visible-light mediated N-dealkylative addition of tertiary amines to dimethyl acetylenedicarboxylates (DMAD) using alkyl halogenides as external oxidants together with base additives. This method is highlighted by excellent tolerance with various tertiary amines and good to excellent yields in short reaction time under mild conditions.
-
Visible-Light-Mediated Dealkylative Coupling of Trialkylamines with Dialkyl Acetylenedicarboxylates作者:Zhiguo Zhang、Fangnan Liu、Zongbi Bao、Baogen Su、Huabin Xing、Qiwei Yang、Yiwen Yang、Qilong RenDOI:10.1055/s-0036-1588704日期:——A dealkylative addition of trialkylamines to dialkyl acetylenedicarboxylates with eosin Y as a metal-free catalyst and molecular oxygen as the terminal oxidant was developed. This visible-light-mediated organocatalytic protocol is compatible with a wide variety of trialkylamines, giving the corresponding adducts in moderate to good yields.
-
Methods for treating an inflammatory condition or inhibiting JNK申请人:——公开号:US20040127536A1公开(公告)日:2004-07-01This invention is generally directed to Indazole Derivatives having the following structure: 1 or pharmaceutically acceptable salt thereof, wherein R 1 , R 2 and A are as defined herein. Such compounds have utility in the treatment of a wide range of diseases and disorders that are responsive to JNK inhibition, such as an inflammatory disease or disorder. Thus, methods of treating such diseases and disorders are also disclosed, as are pharmaceutical compositions containing one or more compounds of the above compounds.
-
Indazole derivatives as JNK inhibitors and compositions and methods related thereto申请人:——公开号:US20020103229A1公开(公告)日:2002-08-01Compounds having activity as selective inhibitors of JNK are disclosed. The compounds of this invention are indazole derivatives having the following structure: 1 wherein R 1 , R 2 and A are as defined herein. Such compounds have utility in the treatment of a wide range of conditions that are responsive to JNK inhibition. Thus, methods of treating such conditions are also disclosed, as are pharmaceutical compositions containing one or more compounds of the above compounds.
-
一种盐酸利多卡因的制备方法申请人:浙江汇能生物股份有限公司公开号:CN105294477B公开(公告)日:2018-01-19本发明提供了一种盐酸利多卡因的制备方法,属于麻醉剂合成技术领域。所述制备方法采用2.6‑二甲酚为原料,采用Pd/C为主催化剂,2,6‑二甲基环己酮为助催化剂,高温下用氨水进行液相胺化得到中间体2,6‑二甲基苯胺;再采用甲醇钠、2,6‑二甲基苯胺和N,N‑二乙氨基乙酸甲酯为原料,于90‑95℃下反应,边反应边蒸馏去除甲醇直至其无甲醇蒸出后,继续反应30min,冷却至室温,加入二氯乙烷,经水洗、静置分层后,有机层即为利多卡因碱的二氯乙烷溶液;向多卡因碱的二氯乙烷溶液中加入盐酸,再以氯化氢调节PH为3.5‑4,加入活性炭回流20‑40min,过滤,滤液经浓缩,冷却结晶,干燥,得盐酸利多卡因。本发明所述的盐酸利多卡因合成工艺简单,产品纯度高达99%以上,总收率高达84%以上。
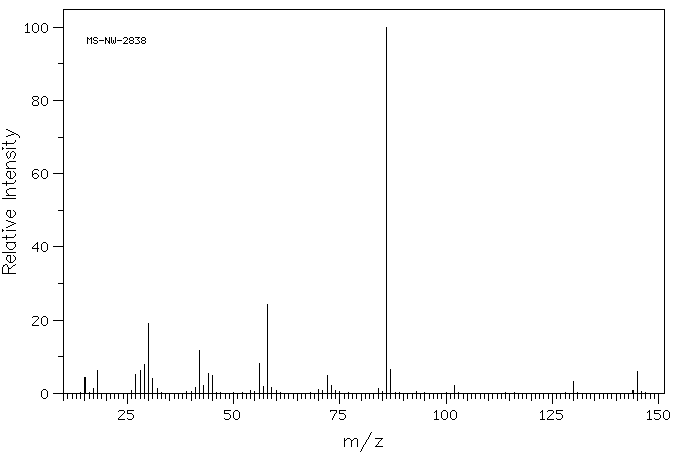
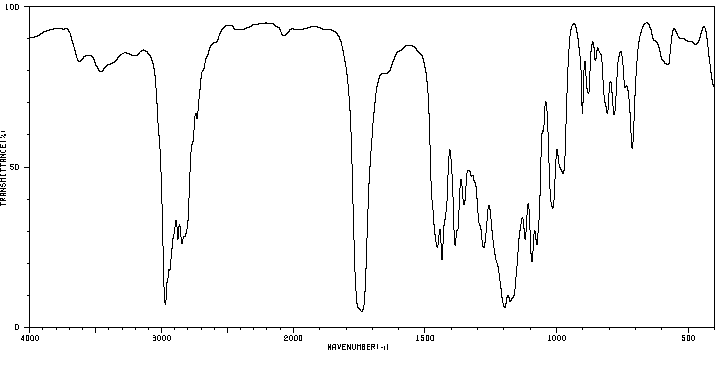
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸