毒理性
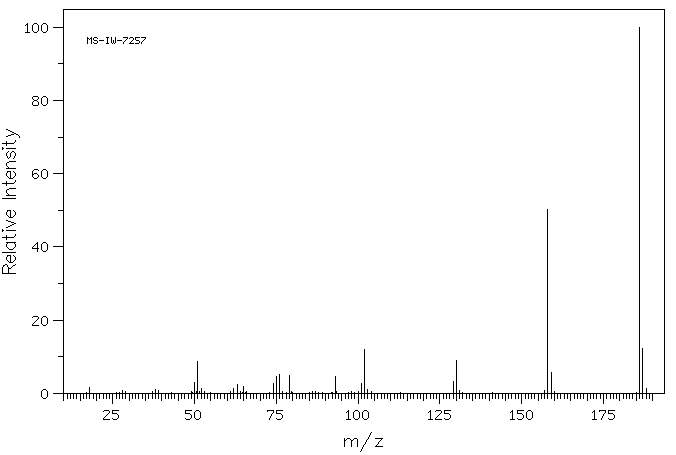
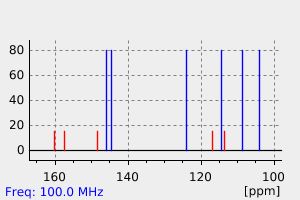
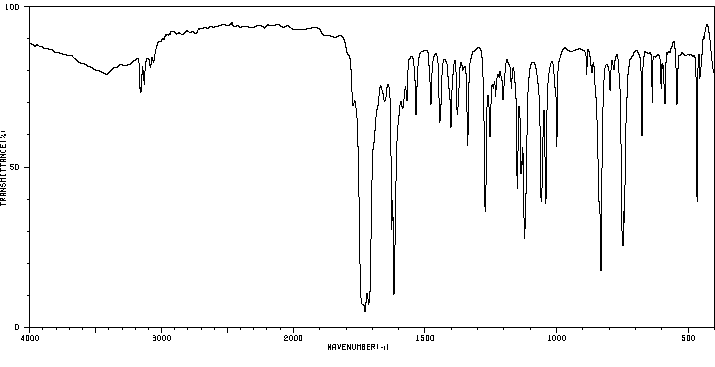
识别和使用:异补骨脂素是一种天然呋喃香豆素。它已被测试为实验性治疗。人体研究:异补骨脂素在体外作为ERalpha受体激动剂,显著促进了MCF-7细胞增殖。当光激活时,当归素形成DNA单加合物。在人类细胞中,异补骨脂素促进了姐妹染色单体交换。动物研究:异补骨脂素在大鼠、小鼠和家兔中通过腹腔注射和口服给药时,具有强大的镇静、抗惊厥和中央肌肉松弛作用。异补骨脂素在细菌和动物细胞中具有光致突变性,生成DNA单加合物。
IDENTIFICATION AND USE: Isopsoralen is a natural furocoumarin. It has been tested as experimental therapy. HUMAN STUDIES: Isopsoralen acted in vitro as ERalpha receptor agonists and promoted MCF-7 cell proliferation significantly. Angelicin forms DNA monoadducts when photoactivated. In human cells isopsoralen promoted sister-chromatid exchanges. ANIMAL STUDIES: Isopsoralen had potent tranquilizing, anticonvulsant, and central muscle relaxant activity in rats, mice, and rabbits when given both ip and orally. Isopsoralen was photomutagenic in bacterial and animal cells generating DNA monoadducts.
来源:Hazardous Substances Data Bank (HSDB)