D-环丝氨酸 | 68-41-7
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:147 °C (dec.)(lit.)
-
比旋光度:111 º (C=5, 2N NaOH)
-
沸点:191.38°C (rough estimate)
-
密度:1.3516 (rough estimate)
-
溶解度:水中的溶解度:50mg/mL,澄清,无色至淡黄色
-
物理描述:Solid
-
颜色/状态:CRYSTALS
-
气味:ODORLESS OR HAS FAINT ODOR
-
稳定性/保质期:
UNSTABLE @ ROOM TEMP; STABLE @ 4-5 °C.
-
旋光度:SPECIFIC OPTICAL ROTATION: +116 DEG @ 23 °C/D (1.17%); +137 DEG @ 25 °C/546 NM (2 N NAOH, 5%)
-
折光率:INDEX OF REFRACTION: 1.583 (ALPHA), 1.630 (GAMMA)
计算性质
-
辛醇/水分配系数(LogP):-1.5
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:64.4
-
氢给体数:2
-
氢受体数:3
ADMET
安全信息
-
危险品标志:Xn
-
安全说明:S24/25
-
危险类别码:R5
-
WGK Germany:2
-
海关编码:2934999090
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
SDS
模块 1. 化学品
1.1 产品标识符
: D-环丝氨酸
产品名称
1.2 鉴别的其他方法
(R)-4-Amino-3-isoxazolidone
4-Amino-3-isoxazolidinone
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: (R)-4-Amino-3-isoxazolidone
别名
4-Amino-3-isoxazolidinone
: C3H6N2O2
分子式
: 102.09 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
避免粉尘生成。 避免吸入蒸气、烟雾或气体。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
建议的贮存温度: -20 °C
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
联合国运输名称: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
联合国运输名称: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所选择身体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
颜色: 白色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 147 °C - 分解
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - > 5,000 mg/kg
备注: 行为的:嗜睡(全面活力抑制)。
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: NY2975000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 16. 其他信息
进一步信息
版权所有:2013 Co. LLC. 公司。许可无限制纸张拷贝,仅限于内部使用。
上述信息视为正确,但不包含所有的信息,仅作为指引使用。本文件中的信息是基于我们目前所知,就正
确的安全提示来说适用于本品。该信息不代表对此产品性质的保证。
参见发票或包装条的反面。
模块 15 - 法规信息
N/A
制备方法与用途
D-环丝氨酸是一种由淡紫灰链霉菌(Streptomyces lavendulae)或兰花链霉菌(S. orchidaceus)产生,也可通过化学合成获得的多肽类广谱抗生素。为白色结晶状物质,吸湿性强,易溶于水,可溶于低级醇、丙酮与二氧六环中,难溶于氯仿和石油醚;在碱性溶液中较为稳定,在酸性和中性溶液中则迅速分解。
D-环丝氨酸的抗菌谱广泛,除了对结核杆菌有抑制作用外,还对大多数革兰阳性及阴性细菌、立克次体以及某些原虫等均有抑制效果。它特别适用于耐药结核杆菌(如链霉素、紫霉素、对氨基水杨酸、异烟肼和吡嗪酰胺耐药的结核杆菌)的治疗。环丝氨酸与异烟肼对结核杆菌H37RV有轻度协同作用,而与其他抗结核药物无明显协同或拮抗作用。
作为抑菌剂,D-环丝氨酸增加剂量或延长作用时间不会产生杀菌效果。其抗菌机制在于抑制细胞壁肽聚糖的生物合成,因其是D-丙氨酸的结构类似物,可与D-丙氨酸竞争性地抑制肽聚糖合成过程中的两个重要酶——丙氨酸消旋酶和D-丙氨酰-D-丙氨酸合成酶。对结核分枝杆菌(Mycobacterium tuberculosis)的抗菌能力较弱,仅为链霉素的1/10~1/20。
环丝氨酸作为二线抗结核药物,虽能抑制结核杆菌生长,但作用相对一线药较弱,对抗结核病的效果亦较低。单独使用可能会产生耐药性,但其耐药性发生缓慢,与其他抗结核药物之间无交叉耐药性。
D-环丝氨酸的化学性质为无色针状或叶状结晶或无定形粉末,熔点155-156℃(分解),溶于水,微溶于甲醇、乙醇、丁醇、丙二醇和异丙醇中,难溶或不溶于甲苯、氯仿、乙醚、吡啶、苯和二硫化碳。
用途方面,D-环丝氨酸主要用于耐药性结核杆菌的感染治疗,并可用于生化研究、酶抑制剂以及通过抑制细胞壁合成(如D-丙氨酸肽键形成)来防止D-丙氨酸转化为L-丙氨酸的过程。
生产方法上,环丝氨酸可通过发酵法或直接合成法制备。发酵法使用赖氏放线菌(Actinomyces laven-dulae),其发酵培养基含有糊精、葡萄糖、淀粉、黄豆饼粉、酵母粉、硫酸铵、硝酸铵、碳酸钙、氯化钠、硫酸镁和豆油等成分;而合成法则通过将β-氨基氧丙氨酸乙酯二盐酸盐与氢氧化钾反应,最终环合得到环丝氨酸。
反应信息
-
作为反应物:参考文献:名称:有效且简便地合成基本不含潜在杂质的D-环丝氨酸摘要:已经从D-丝氨酸开发了一种有效且容易的D-环丝氨酸合成方法,采用保护脱保护策略,总收率达61%。研究了影响D-环丝氨酸杂质含量和产率的不同参数。温和的反应条件为产品提供了卓越的纯度(> 99%)和高稳定性。DOI:10.1007/s10593-018-2197-y
-
作为产物:描述:methyl (2R)-2-amino-3-(aminooxy)propanoate 在 sodium hydroxide 、 溶剂黄146 作用下, 以 水 、 异丙醇 为溶剂, 反应 4.0h, 以80%的产率得到D-环丝氨酸参考文献:名称:有效且简便地合成基本不含潜在杂质的D-环丝氨酸摘要:已经从D-丝氨酸开发了一种有效且容易的D-环丝氨酸合成方法,采用保护脱保护策略,总收率达61%。研究了影响D-环丝氨酸杂质含量和产率的不同参数。温和的反应条件为产品提供了卓越的纯度(> 99%)和高稳定性。DOI:10.1007/s10593-018-2197-y
-
作为试剂:描述:参考文献:名称:固体NMR光谱分析Pradimicin A的Ca2 +依赖性甘露糖结合摘要:聚集促进了分析:固态研究了非肽类碳水化合物结合剂radimicin A(PRM-A)的Ca 2+依赖性甘露糖(Man)结合。PRM-A聚合的使用消除了与三成分均衡相关的问题。的组合113镉NMR光谱和2D偶极辅助旋转共振揭示PRM-A的甘露糖结合位点和Ca的关键作用2+离子(见结合模型)。DOI:10.1002/anie.201007775
文献信息
-
Cyclic hydroxamates, especially multiply substituted [1,2]oxazinan-3-ones作者:Saul Wolfe、Marie-Claire Wilson、Ming-Huei Cheng、Gennady V Shustov、Christiana I AkucheDOI:10.1139/v03-122日期:2003.8.1
Routes to putative N-acyl-D-ala-D-ala surrogates, beginning with the conversion of 4-, 5-, and 6-membered lactones into 5-, 6-, and 7-membered cyclic hydroxamates, are reported. The key step of the synthesis is trimethylaluminium-promoted cyclization of an ω-aminooxyester. The 7-membered cyclic hydroxamate crystallizes in a chair conformation. Extension of the reaction sequence to homoserine or homoserine lactone leads to cyclocanaline and N-acylated cyclocanalines. The 4-phenylacetamido derivative of cyclocanaline crystallizes in a boat conformation. The attachment of a 2-carboxypropyl substituent to the ring nitrogen of a 4-acylaminocyclocanaline has been effected, prior to cyclization, by coupling of the acyclic aminooxyester precursor to the triflate of benzyl lactate or, after cyclization, by coupling to tert-butyl α-bromopropionate in the presence of potassium fluoride alumina, followed by removal of the protecting group in each case. A six-membered homolog of the antibiotic lactivicin has been synthesized by the reaction of 4-phenylacetamidocyclocanaline with benzyl 2-oxoglutarate in the presence of carbodiimide, followed by hydrogenolysis. Starting with methyl 2,4-dibromo-2,4-dideoxy-L-erythronate, which is available in two steps from L-ascorbic acid, these reaction sequences have been applied to the stereospecific synthesis of a D-alanine derivative whose nitrogen atom is enclosed within a 3,4-disubstituted [1,2]oxazinan-3-one. Key words: D-ala-D-ala surrogate, cyclocanaline, homolactivicin, peptidoglycan, trimethylaluminium.
报道了将假定的N-酰-D-阿拉-D-阿拉替代物的合成途径,从将4、5和6元环内酯转化为5、6和7元环氢氧化物开始。合成的关键步骤是ω-氨氧酯的三甲基铝促进的环化反应。7元环氢氧化物以椅式构象结晶。将反应序列扩展到同型丝氨酸或同型丝氨酸内酯会导致环康纳林和N-酰化环康纳林的形成。环康纳林的4-苯乙酰氨基衍生物以船式构象结晶。在环康纳林的4-酰氨基环康纳林的环氮上附加2-羧基丙基取代基,可以通过将无环氨氧酯前体与苄乳酸三甲酯的三氟甲磺酸酯偶联,或在环化之后,通过与三氟甲基丙酸叔丁酯在氟化钾-氧化铝存在下偶联,然后在每种情况下去除保护基来实现。通过4-苯乙酰氨基环康纳林与苄基2-氧戊二酸酯在异亚胺存在下反应,然后进行氢解,合成了抗生素拉维西汀的六元同系物。从甲基2,4-二溴-2,4-二去氧-L-赤葡萄糖酸甲酯开始,该物质可以从抗坏血酸中经过两步合成,这些反应序列已被应用于立体特异性合成一种D-丙氨酸衍生物,其氮原子被包含在一个3,4-二取代[1,2]噁唑烷-3-酮内。关键词:D-ala-D-ala替代物,环康纳林,同型拉维西汀,肽聚糖,三甲基铝。 -
Kinase inhibitor compounds申请人:Liang Congxin公开号:US20090076005A1公开(公告)日:2009-03-19The invention relates to compounds, compositions comprising the compounds, and methods of using the compounds and compound compositions. The compounds, compositions, and methods described herein can be used for the therapeutic modulation of kinase-mediated processes, and treatment of disease and disease symptoms, particularly those mediated by certain kinase enzymes.这项发明涉及化合物、包含这些化合物的组合物,以及使用这些化合物和化合物组合物的方法。本文描述的化合物、组合物和方法可用于治疗激酶介导的过程,并治疗疾病和疾病症状,特别是那些由特定激酶酶介导的症状。
-
[EN] INSECTICIDAL COMPOUNDS<br/>[FR] COMPOSÉS INSECTICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2012163959A1公开(公告)日:2012-12-06The present invention provides compounds of formula (I): wherein A1, A2, A3 and A4 are independently of one another C-H, C-R5, or nitrogen; B1--B2--B3--B4 is -CH2-C=N-CH2-, -CH2-N-CH2-CH2-, -CH2-C=CH-0- or -CH=C-CH2-0-; G1 is oxygen or sulfur; L is a single bond or C1-C8alkylene; R1 is hydrogen, C1-C8alkyl, C1-C8alkylcarbonyl-, C1-C8alkoxy, C2-C8alkenyl, C2-C8alkynyl, C1-C8alkoxy- C1-C8alkyl, aryl or aryl substituted by one to three R6, or R1 is heterocyclyl or heterocyclyl substituted by one to three R6 or C1-C8alkoxycarbonyl-; R2 is hydrogen, C1-C8haloalkyl or C1-C8alkyl; R3 is C1-C8haloalkyl; R4 is aryl or aryl substituted by one to three R6, or R4 is heterocyclyl or heterocyclyl substituted by one to three R6; Y1 is CR7R8, C=O or C=S; Y2, Y3 and Y4 are independently CR7R8, C=0, C=S, N-R9, O, S, SO or SO2; wherein at least two adjacent ring atoms in the ring formed by Y1, Y2, Y3 and Y4 are heteroatoms; each R7 and R8 is independently hydrogen, halogen, C1-C8alkyl, or C1-C8haloalkyl; and R5, R6, R7, R8 and R9 are as defined in the claims. The invention also relates to processes and intermediates for preparing these compounds, to insecticidal, acaricidal, nematicidal and molluscicidal compositions comprising these compounds and to methods of using these compounds to control insect, acarine, nematode and mollusc pests.本发明提供了以下式(I)的化合物:其中A1、A2、A3和A4彼此独立地为C-H、C-R5或氮;B1--B2--B3--B4为-CH2-C=N- -、- -N- - -、- -C=CH-0-或-CH=C- -0-;G1为氧或硫;L为单键或C1-C8烷基;R1为氢、C1-C8烷基、C1-C8烷基羰基、C1-C8烷氧基、C2-C8烯基、C2-C8炔基、C1-C8烷氧基-C1-C8烷基、芳基或被1至3个R6取代的芳基,或R1为杂环烷基或被1至3个R6或C1-C8烷氧羰基取代的杂环烷基;R2为氢、C1-C8卤代烷基或C1-C8烷基;R3为C1-C8卤代烷基;R4为芳基或被1至3个R6取代的芳基,或R4为杂环烷基或被1至3个R6取代的杂环烷基;Y1为CR7R8、C=O或C=S;Y2、Y3和Y4独立地为CR7R8、C=0、C=S、N-R9、O、S、SO或SO2;其中由Y1、Y2、Y3和Y4形成的环中至少有两个相邻的环原子为杂原子;每个R7和R8独立地为氢、卤素、C1-C8烷基或C1-C8卤代烷基;而R5、R6、R7、R8和R9如权利要求中所定义。本发明还涉及制备这些化合物的过程和中间体,包括这些化合物的杀虫剂、杀螨剂、杀线虫剂和杀软体动物剂组合物,以及使用这些化合物控制昆虫、螨类、线虫和软体动物害虫的方法。
-
Triazolo[4,5-D]pyrimidinyl derivatives and their use as medicaments
-
INSECTICIDAL COMPOUNDS BASED ON ISOAZOLINE DERIVATIVES申请人:Cassayre Jerome Yves公开号:US20120238517A1公开(公告)日:2012-09-20The present invention relates to compounds of formula (I): Wherein A 1 , A 2 , A 3 , A 4 , G 1 , L, Y 1 , Y 2 , Y 3 , Y 4 , R 1 , R 2 , R 3 and R 4 are as defined in claim 1 ; or a salt or N-oxide thereof. Furthermore, the present invention relates to intermediates for preparing compounds of formula (I), to compositions comprising them and to methods of using them to combat and control insect, acarine, nematode and mollusc pests.本发明涉及式(I)的化合物:其中A1、A2、A3、A4、G1、L、Y1、Y2、Y3、Y4、R1、R2、R3和R4如权利要求1所定义;或其盐或N-氧化物。此外,本发明涉及制备式(I)化合物的中间体,包含它们的组合物以及使用它们来对抗和控制昆虫、螨虫、线虫和软体动物害虫的方法。
表征谱图
-
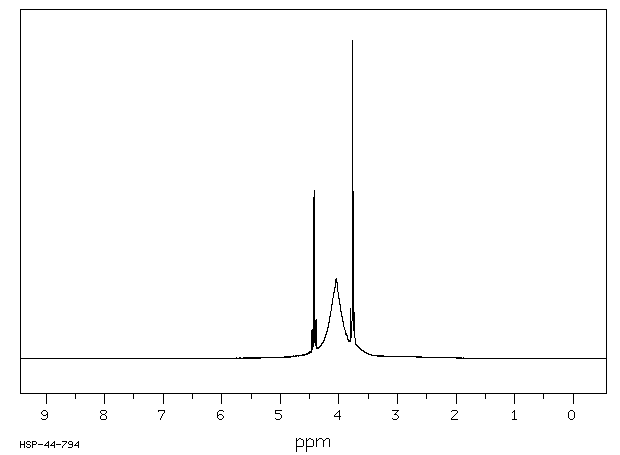
氢谱1HNMR
-
质谱MS
-
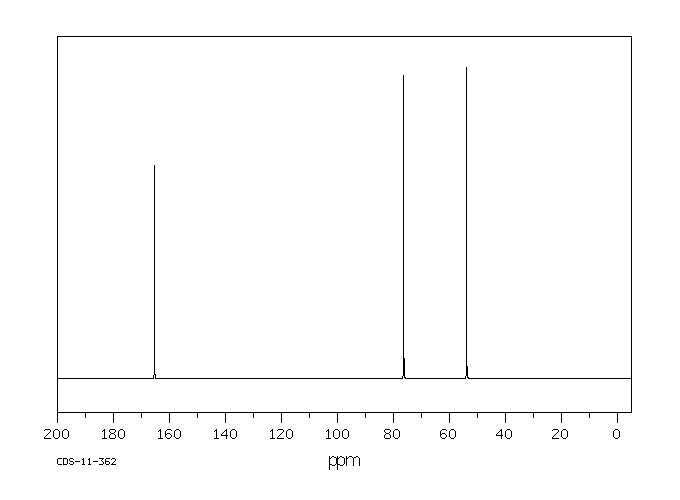
碳谱13CNMR
-
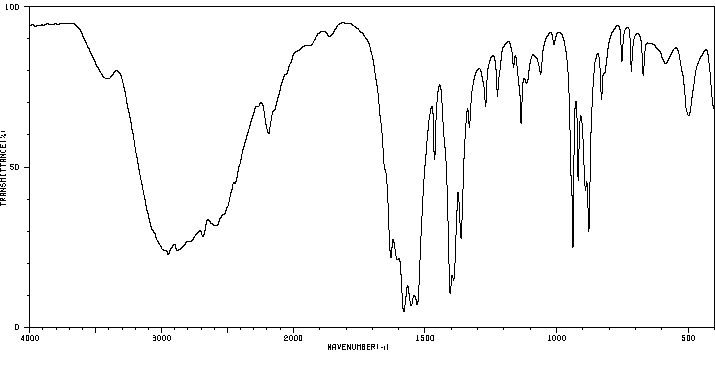
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息