s,s'-三甲烯(p-甲苯硫代磺酸盐) | 3866-79-3
中文名称
s,s'-三甲烯(p-甲苯硫代磺酸盐)
中文别名
三亚甲基二(硫代对甲苯磺酸酯);三甲烯二(甲苯硫代磺酸盐)
英文名称
1,3-bis(p-toluenesulfonylthio)propane
英文别名
1,3-bis(tosylsulfanyl)propane;trimethylene di(thiotosylate);Trimethylene dithiotosylate;1,3-bis-(toluene-4-sulfonylsulfanyl)-propane;1,3-bis-(toluene-4-sulfonylmercapto)-propane;1,3-Bis-(toluol-4-sulfonylmercapto)-propan;1-methyl-4-[3-(4-methylphenyl)sulfonylsulfanylpropylsulfanylsulfonyl]benzene
CAS
3866-79-3
化学式
C17H20O4S4
mdl
MFCD00014918
分子量
416.607
InChiKey
QOICHLMIPNOKLN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:64-67 °C
-
沸点:592.3±60.0 °C(Predicted)
-
密度:1.340±0.06 g/cm3(Predicted)
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:25
-
可旋转键数:8
-
环数:2.0
-
sp3杂化的碳原子比例:0.294
-
拓扑面积:136
-
氢给体数:0
-
氢受体数:6
安全信息
-
安全说明:S22,S24/25
-
WGK Germany:3
-
海关编码:2930909090
SDS
三亚甲基二(硫代对甲苯磺酸酯)[活泼亚甲基的保护试剂] 修改号码:2
模块 1. 化学品
产品名称: Trimethylene Di(thiotosylate) [Protecting ReageNT for Active Methylene]
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 三亚甲基二(硫代对甲苯磺酸酯)[活泼亚甲基的保护试剂]
百分比: >95.0%(LC)
CAS编码: 3866-79-3
俗名: 1,3-Di(p-tosylthio)propane , 1,3-Propanediyl Di(thiotosylate) ,
Trimethylene Bis(p-toluenethiosulfonate)
分子式: C17H20O4S4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
修改号码:2
护试剂]
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点:
66°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
修改号码:2
护试剂]
模块 10. 稳定性和反应性
反应性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
修改号码:2
护试剂]
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Trimethylene Di(thiotosylate) [Protecting ReageNT for Active Methylene]
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 三亚甲基二(硫代对甲苯磺酸酯)[活泼亚甲基的保护试剂]
百分比: >95.0%(LC)
CAS编码: 3866-79-3
俗名: 1,3-Di(p-tosylthio)propane , 1,3-Propanediyl Di(thiotosylate) ,
Trimethylene Bis(p-toluenethiosulfonate)
分子式: C17H20O4S4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
修改号码:2
护试剂]
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点:
66°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
修改号码:2
护试剂]
模块 10. 稳定性和反应性
反应性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
修改号码:2
护试剂]
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:s,s'-三甲烯(p-甲苯硫代磺酸盐) 在 甲基碘化镁 、 乙醚 、 乙醇 、 potassium tert-butylate 、 potassium acetate 、 镍 、 叔丁醇 、 苯 作用下, 生成 3β,4β-dimethyl-5α-cholestan-3α-ol参考文献:名称:Beton et al., Journal of the Chemical Society, 1957, p. 753,761摘要:DOI:
-
作为产物:描述:sodium 4-methylbenzenesulfinate 、 1,3-二溴丙烷 在 吡啶 、 sulfur 作用下, 以 水 、 甲苯 为溶剂, 反应 86.0h, 以97%的产率得到s,s'-三甲烯(p-甲苯硫代磺酸盐)参考文献:名称:环丁烷-1,2-二酮和环丁三酮的稳定等价物的方便的一锅法制备摘要:摘要 已经开发了一种非常短、高产的单锅程序来制备半保护的环丁烷-1,2-二酮。该化合物比环丁烷-1,2-二酮本身稳定得多,并允许进一步转化以得到双保护的环丁三酮等价物。DOI:10.1080/00397910701845308
-
作为试剂:描述:(1S,4R,5R)-4-prop-2-enyl-2-oxabicyclo[3.2.1]octan-3-one 在 四氢吡咯 、 Jones's reagent 、 s,s'-三甲烯(p-甲苯硫代磺酸盐) 作用下, 生成 (R)-2-((R)-4-Oxo-6,10-dithia-spiro[4.5]dec-2-yl)-pent-4-enoic acid ethyl ester参考文献:名称:Takano, Seiichi; Morimoto, Masamichi; Masuda, Ken'ichi, Chemical and pharmaceutical bulletin, 1982, vol. 30, # 11, p. 4238 - 4241摘要:DOI:
文献信息
-
An approach to modified heterocyclic analogues of huperzine A and isohuperzine A. Synthesis of the pyrimidone and pyrazole analogues, and their anticholinesterase activity作者:Alan P. Kozikowski、Giuseppe Campiani、Vito Nacci、Alessandro Sega、Ashima Saxena、Bhupendra P. DoctorDOI:10.1039/p19960001287日期:——and the pyrazole analogues of the naturally occurring acetylcholinesterase (AChE) inhibitor huperzine A and its unnatural regioisomer, isohuperzine, are described. The pyrimidone analogues of huperzine A were obtained starting from cyclohexane-1,4-dione monoethylene ketal by first annealing to this monocycle a pyrimidine ring and then constructing the unsaturated three-carbon bridge using the previously描述了天然存在的乙酰胆碱酯酶(AChE)抑制剂石杉碱A及其非天然区域异构体异石杉碱的嘧啶酮和吡唑类似物的合成方法。石杉碱甲的嘧啶酮类似物是从环己烷-1,4-二酮单亚乙基缩酮开始,首先退火至该单环一个嘧啶环,然后使用前述的钯催化双环化方法构建的不饱和三碳桥基。事实证明,该合成过程中的主要问题是将亚乙基附属物引入三轮车10。尽管Wittig和Takai烯烃化方案均未成功,最终使用Danheiser方法引入亚乙基部分,该方法涉及两步反应序列,该过程由β-内酯的中间结构组成,该结构继而经历[2 + 2]环还原反应,从而生成所需的烯烃。已经发现这种先前未应用于β-酮酯的β-内酯合成以优异的非对映选择性进行。反过来,β-内酯进行立体有择的脱羧反应以提供作为唯一异构体的E-烯烃产物。此外,从双环[3.3.1]壬烯中间体2开始,我们描述了一种合成策略,用于购买异石杉碱A的修饰杂环类似物。该化学方法提供了一种诱人的方法,用于合成6
-
Synthetic studies relevant to biosynthetic research on vitamin B12. Part 1. Syntheses of C-methylated chlorins based on 1-pyrrolines (3,4-dihydropyrroles)作者:Alan R. Battersby、Christopher J. R. Fookes、Roger J. SnowDOI:10.1039/p19840002725日期:——Two routes are explored for the synthesis of chlorins geminally substituted in the reduced ring; both involve cyclisations of 1-pyrroline (3,4-dihydropyrrole) systems promoted by copper(II) salts. In the preferred synthesis, a pyrrolomethyl-1-pyrroline is combined with a 5-bromo-5′-bromomethyl-pyrromethene; though not high yielding, this approach involves few steps from readily prepared building blocks
-
A Search for New Synthetic Routes toward Siccanin作者:Michiharu Kato、Yukio Matsumura、Kiyoshi Heima、Akira YoshikoshiDOI:10.1246/bcsj.61.1991日期:1988.611-Acetoxy-12bα-methoxymethyl-4,4,6aα,9-tetramethyl-1,2,3,4,4aα,5,6,6a, 12aβ,12b-decahydro-12H-benzo[a]xanthene (34), which has been expected as a promising key intermediate for the synthesis of siccanin (1), was synthesized via acid-catalyzed cyclization of 1α-(2-hydroxy-6-methoxy-4-methylbenzyl)-8aα-methoxymethyl-2,5,5-trimethyl-1,4,4aα,5,6,7,8,8aα-octahydronaphthalene (13) and isomeric exo olefin 21. The phenol acetate 34, however, was so resistant to oxidation that no corresponding 12-oxo derivative, which was expected to provide 11-acetoxy-12bα-acetoxymethyl-12-oxo-4,4,6aα,9-tetramethyl-1,2,3,4,4aα,5,6,6a,12aα,12b-decahydro-12H-benzo[a]xanthene (4) on epimerization at its C(12a) position as well as hydrofuran ring formation at the C(12) position with its angular methoxymethyl substituent, was obtained.11-乙酰氧基-12bα-甲氧甲基-4,4,6aα,9-四甲基-1,2,3,4,4aα,5,6,6a,12aβ,12b-十氢-12H-苯并[a]黄烷 (34),被预计为合成西卡宁 (1) 的一个有前景的关键中间体,通过 1α-(2-羟基-6-甲氧基-4-甲基苯基)-8aα-甲氧甲基-2,5,5-三甲基-1,4,4aα,5,6,7,8,8aα-八氢萘 (13) 与异构体外烯烃 21 的酸催化环化反应合成。然而,酚醋酸酯 34 对氧化的抗性极强,以至于未获得相应的 12-氧基衍生物,该衍生物预计在其 C(12a) 位点通过表异构化和在 C(12) 位点形成含有角基甲氧基甲基取代基的氢呋喃环,将生成 11-乙酰氧基-12bα-乙酰氧基甲基-12-氧基-4,4,6aα,9-四甲基-1,2,3,4,4aα,5,6,6a,12aα,12b-十氢-12H-苯并[a]黄烷 (4)。
-
Synthesis of 1,2,4-triazines and the triazinoisoquinolinedione DEF ring system of noelaquinone作者:Liming Cao、John P. Maciejewski、Stephan Elzner、David Amantini、Peter WipfDOI:10.1039/c2ob25353d日期:——The intramolecular Staudinger-aza-Wittig reaction is used for a general synthesis of 1,2,5,6-tetrahydro-1,2,4-triazines, a structural motif reported for the natural product noelaquinone. The DEF moiety of noelaquinone was obtained in 13 steps and 2% overall yield, and the structure of the synthetic product was confirmed by X-ray analysis.
-
New and Facile Synthesis of Thiosulfonates from Sulfinate/Disulfide/I2 System作者:Kiyoko Fujiki、Naoki Tanifuji、Yohei Sasaki、Taku YokoyamaDOI:10.1055/s-2002-20031日期:——Thiosulfonates were prepared by the iodine oxidative sulfenylation of sulfinates with various disulfides in good yields both in the presence and absence of solvent. One of the important biological applications of sulfenylation is the reaction of cyclic disulfides.
表征谱图
-
氢谱1HNMR
-
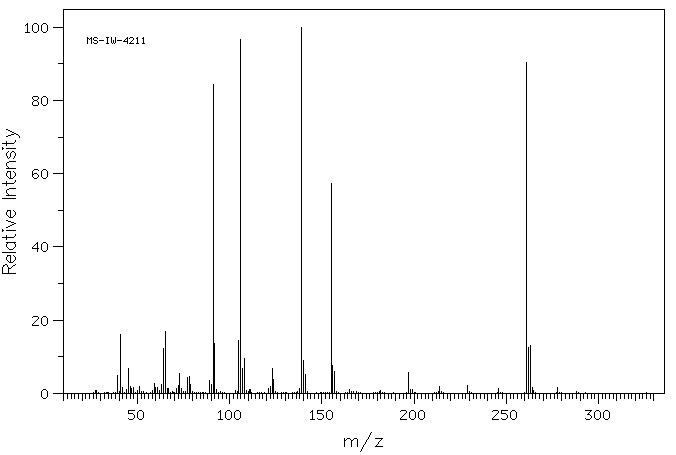
质谱MS
-
碳谱13CNMR
-
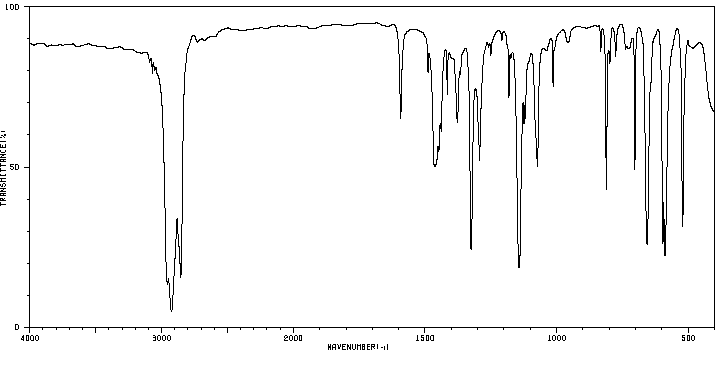
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫