十氟二苯甲醇 | 1766-76-3
中文名称
十氟二苯甲醇
中文别名
十甲基苯氟酸酯
英文名称
decafluorobenzhydrol
英文别名
1,1-bis(pentafluorophenyl)methanol;Bis(pentafluorophenyl)methanol;bis(2,3,4,5,6-pentafluorophenyl)methanol
CAS
1766-76-3
化学式
C13H2F10O
mdl
MFCD00000297
分子量
364.142
InChiKey
WRLLBTKDSCJOBL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:77-80 °C(lit.)
-
沸点:110 °C / 1.5mmHg
-
密度:1.6108 (estimate)
-
稳定性/保质期:
常温常压下稳定,可与氧化物发生反应。
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:24
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.08
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:11
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S22,S24/25
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2906299090
-
危险类别:IRRITANT
SDS
| Name: | Decafluorobenzhydrol 97+% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 1766-76-3 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1766-76-3 | Decafluorobenzhydrol | 97+ | 217-185-0 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1766-76-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: off-white
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 77.00 - 80.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C13H2F10O
Molecular Weight: 364.13
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Acids, acid chlorides, acid anhydrides, oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen fluoride gas.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1766-76-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Decafluorobenzhydrol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1766-76-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1766-76-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1766-76-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 全氟二苯甲酮 bis(pentafluorophenyl)-methanone 853-39-4 C13F10O 362.126 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 二(五氟苯基)甲烷 bis(pentafluorophenyl)methane 5736-46-9 C13H2F10 348.143 全氟二苯甲酮 bis(pentafluorophenyl)-methanone 853-39-4 C13F10O 362.126 1,1'-(溴亚甲基)二[2,3,4,5,6-五氟苯] 1,1'-(Bromomethylene)bis[2,3,4,5,6-pentafluorobenzene] 5736-49-2 C13HBrF10 427.039 —— 1,1-Bis(pentafluorophenyl)-3,3-dichloropropene —— C15H2Cl2F10 443.071
反应信息
-
作为反应物:描述:参考文献:名称:未活化 α-支化硝基烷烃的有机催化迈克尔加成得到光学活性叔硝基化合物摘要:未活化 α-支化硝基烷烃的直接、不对称共轭加成是基于手性胺/脲醛胺双功能催化剂和可调丙烯酸酯模板的组合使用而开发的,可提供 55-80% 分离产率和高对映选择性(呃高达 96)的叔硝基化合物。 :4)。由此获得的加合物中酮醇部分的精制不仅可以快速进入羧酸和醛衍生物,还可以快速进入腈化合物和对映体富集的5,5-二取代的γ-内酰胺。DOI:10.1021/acs.orglett.3c03340
-
作为产物:参考文献:名称:173.芳族多氟化合物。第八部分 五氟苯甲醛及相关的五氟苯基酮和羧酸摘要:DOI:10.1039/jr9610000808
文献信息
-
H<sub>2</sub> activation by a highly electron-deficient aralkylated organoborane作者:Peter J. Hill、Thomas J. Herrington、Nicholas H. Rees、Andrew J. P. White、Andrew E. AshleyDOI:10.1039/c5dt00821b日期:——The electron-deficient and sterically bulky trialkylborane derivative tris[bis(pentafluorophenyl)methyl]borane [1, B(CH(C6F5)2)3], has been synthesised and comprehensively characterised; detailed 1H and 19F NMR studies reveal two dynamic bond rotational processes in the solution phase. Despite conventional probes (Gutmann–Beckett and Childs methods) implying that the compound has a very limited Lewis
-
Diastereoselective hydroalkylation of aryl alkenes enabled by Remote hydride transfer作者:Dhika Aditya Gandamana、Fabien Gagosz、Shunsuke ChibaDOI:10.1016/j.tet.2020.131272日期:2020.12Leveraging of 1,5-hydride shift enabled diastereoselective hydroalkylation of aryl alkenes, allowing for construction of consecutive vicinal stereogenic centers in a single process. The transformation is triggered by electrophilic alkylation of aryl alkenes with in-situ generated carbocations, that is followed by diastereoselective 1,5-hydride shift to the resulting benzylic carbocation through a rigid
-
[EN] BISINDENYL ZIRCONIUM COMPLEXES FOR USE IN THE POLYMERISATION OF OLEFINS<br/>[FR] COMPLEXES DE ZIRCONIUM DE BISINDENYLE UTILISABLES POUR LA POLYMERISATION D'OLEFINES申请人:BASELL POLYOLEFINE GMBH公开号:WO2004106351A1公开(公告)日:2004-12-09The present invention relates to organometallic transition metal compounds of the formula (I); biscyclopentadienyl ligand systems having such a substitution pattern, catalyst systems comprising at least one of the organometallic transition metal compounds of the present invention, a process for preparing polyolefins by polymerization or copolymerization of at least one olefin in the presence of one of the catalyst systems of the present invention and also the use of the biscyclopentadienyl ligand systems of the present invention for preparing organometallic transition metal compounds.
-
Kinetic hydricity of silane hydrides in the gas phase作者:Jiahui Xu、Allison E. Krajewski、Yijie Niu、G. S. M. Kiruba、Jeehiun K. LeeDOI:10.1039/c9sc02118c日期:——Herein, gas phase studies of the kinetic hydricity of a series of silane hydrides are described. An understanding of hydricity is important as hydride reactions figure largely in many processes, including organic synthesis, photoelectrocatalysis, and hydrogen activation. We find that hydricity trends in the gas phase differ from those in solution, revealing the effect of solvent. Calculations and further
-
[EN] MONOCYCLOPENTADIENYL COMPLEXES<br/>[FR] COMPLEXES DE MONOCYCLOPENTADIENYLE申请人:BASELL POLYOLEFINE GMBH公开号:WO2005058928A1公开(公告)日:2005-06-30Monocyclopentadienyl complexes in which the cyclopentadienyl system bears at least one bridged donor and at least one aryl group and a catalyst system comprising at least one of the monocyclopentad ienyl complexes, and also processes for preparing them, the use of the catalyst system for the polymerization or copolymerization of olefins and a process for preparing polyole fins by polymerization or copolymerization of olefins in the presence of the catalyst system and the preparation of the associated cyclopentadienyl system.
表征谱图
-
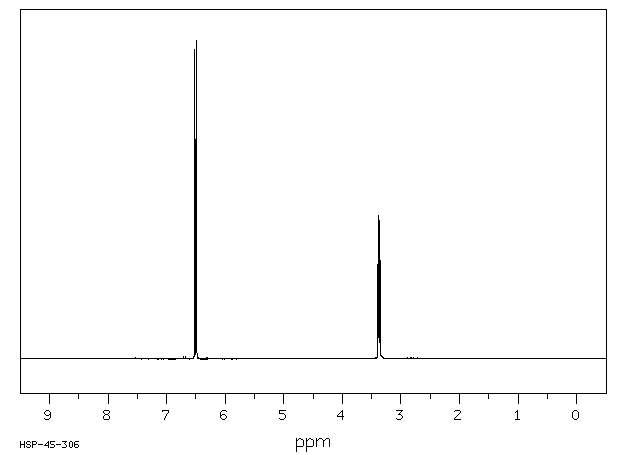
氢谱1HNMR
-
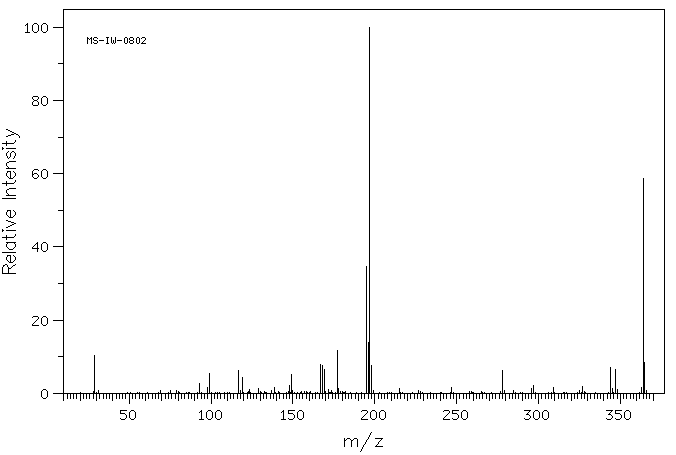
质谱MS
-
碳谱13CNMR
-
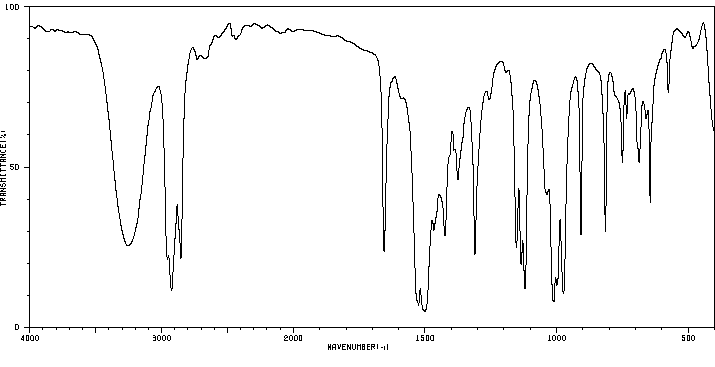
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫