2,4-二溴-5-甲氧基甲苯 | 5456-94-0
中文名称
2,4-二溴-5-甲氧基甲苯
中文别名
1,5-二溴-2-甲氧基-4-甲基苯
英文名称
2,4-dibromo-5-methylanisole
英文别名
2,4-dibromo-5-methyl-anisole;2,4-Dibrom-5-methyl-anisol;Methyl-(4.6-dibrom-3-methyl-phenyl)-aether;4.6-Dibrom-3-methoxy-toluol;1,5-dibromo-2-methoxy-4-methyl-benzene;2,4-Dibrom-5-Methoxytoluol;2,4-Dibromo-5-methoxytoluene;1,5-dibromo-2-methoxy-4-methylbenzene
CAS
5456-94-0
化学式
C8H8Br2O
mdl
——
分子量
279.959
InChiKey
CDLNHQVKSMDSAI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:74-76°C
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:IRRITANT
-
海关编码:2909309090
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2,4-Dibromo-5-methylanisole
Synonyms: 2,4-Dibromo-5-methoxytoluene; 1,5-Dibromo-2-methoxy-4-methylbenzene
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2,4-Dibromo-5-methylanisole
CAS number: 5456-94-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H8Br2O
Molecular weight: 280.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2,4-Dibromo-5-methylanisole
Synonyms: 2,4-Dibromo-5-methoxytoluene; 1,5-Dibromo-2-methoxy-4-methylbenzene
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2,4-Dibromo-5-methylanisole
CAS number: 5456-94-0
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H8Br2O
Molecular weight: 280.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,4-二溴-5-甲基苯酚 2,4-dibromo-5-methylphenol 13321-76-1 C7H6Br2O 265.932 4-溴-3-甲基苯酚 4-bromo-3-methylphenol 14472-14-1 C7H7BrO 187.036 5-溴-4-甲氧基-2-甲基-苯胺 5-bromo-4-methoxy-2-methylaniline 861084-04-0 C8H10BrNO 216.077 5-溴-2-甲氧基-4-甲基苯胺 5-bromo-2-methoxy-4-methyl-aniline 808133-98-4 C8H10BrNO 216.077 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2,4-Dibrom-5-methoxybenzylbromid 125714-92-3 C8H7Br3O 358.855 2,4-二溴-5-甲基苯酚 2,4-dibromo-5-methylphenol 13321-76-1 C7H6Br2O 265.932 2-溴-5-甲氧基甲苯 4-bromo-3-methylanisole 27060-75-9 C8H9BrO 201.063 2,3,6-三溴对甲酚 2,4,6-tribromo-m-cresol 4619-74-3 C7H5Br3O 344.828 —— 3-(2',4'-Dibrom-5'-methoxyphenyl)propionsaeure 6520-93-0 C10H10Br2O3 337.996 5-溴-2-甲氧基-4-甲基-苯酚 5-bromo-2-methoxy-4-methylphenol 83387-13-7 C8H9BrO2 217.062
反应信息
-
作为反应物:描述:参考文献:名称:Haworth; Lapworth, Journal of the Chemical Society, 1923, vol. 123, p. 2989摘要:DOI:
-
作为产物:描述:参考文献:名称:可见光照射下有机-无机杂化钙钛矿基光催化剂的芳香溴化产氢摘要:芳香族溴化物是自然界和化学工业中的重要化学品。然而,它们的传统合成路线存在原子经济性低和污染物形成的问题。在此,我们展示了在 HBr 水溶液中稳定的有机-无机杂化钙钛矿甲基溴化铅 (MAPbBr 3 ) 纳米晶体可以在可见光照射下使用 HBr 作为溴源同时实现芳香族溴化和析氢。通过将 MAPbBr 3与 Pt/Ta 2 O 5和聚(3,4-乙烯二氧噻吩)聚苯乙烯磺酸盐作为电子和空穴传输基序杂化,以高产率(高达 99%)和选择性(高达 99%) 与添加N, N-二甲基甲酰胺或其类似物。机理研究表明溴化通过亲电攻击途径进行,HOBr可能是溴化反应的关键中间体。DOI:10.1016/s1872-2067(22)64101-9
文献信息
-
Dehydroxymethyl Bromination of Alkoxybenzyl Alcohols by Using a Hypervalent Iodine Reagent and Lithium Bromide作者:Tomohiro Maegawa、Ayako Shibata、Sara Kitamoto、Kazuma Fujimura、Yuuka Hirose、Hiromi Hamamoto、Akira Nakamura、Yasuyoshi MikiDOI:10.1055/s-0037-1610980日期:2018.10We describe the dehydroxymethylbromination of alkoxybenzyl alcohol by using a hypervalent iodine reagent and lithium bromide in F3CCH2OH at room temperature. Selective monobromination or dibromination was possible by adjusting the molar ratios of hypervalent iodine reagent and lithium bromide.
-
Effect of Cyclodextrins on Electrophilic Aromatic Bromination in Aqueous Solution作者:Paul G. Dumanski、Christopher J. Easton、Stephen F. Lincoln、Jamie S. SimpsonDOI:10.1071/ch03102日期:——molecular reactors to change the ratios of the products of reactions of anisole, acetanilide, 3-methylanisole, and 3-methylacetanilide with pyridinium dichlorobromate. With anisole and acetanilide, bromination at the para position is favoured over ortho substitution, and the effect is greatest with α-cyclodextrin. In the reactions of the methylanisole and methylacetanilide, the cyclodextrins afford higher环糊精充当分子反应器以改变苯甲醚、乙酰苯胺、3-甲基苯甲醚和3-甲基乙酰苯胺与二氯溴酸吡啶鎓的反应产物的比率。对于苯甲醚和乙酰苯胺,对位溴化优于邻位取代,α-环糊精效果最大。在甲基苯甲醚和甲基乙酰苯胺的反应中,环糊精的一溴化物产率较高,二溴化物和三溴化物的产率较低,β-环糊精的效果最大。这些结果可归因于在环糊精中包含底物,限制了与甲氧基和乙酰氨基基团相邻的试剂的接近。4-溴苯甲醚、4-溴乙酰苯胺、4-溴-3-甲基苯甲醚和4-溴-3-甲基乙酰苯胺的产率因此从73增加到94,分别为 55 至 98、37 至 86 和 39 至 72%。或许更重要的是,相应副产品的数量大幅减少,从 27% 减少到 6%,从 45% 减少到 2%,从 63% 减少到 14%,从 61% 减少到 28%。由于反应在环境温度下很容易在水中发生,环糊精使它们非常有效。
-
THE SYNTHESIS OF TWO ISOMERIC THYMOLS作者:R. A. B. Bannard、L. C. LeitchDOI:10.1139/v56-188日期:1956.10.1
2-Isopropyl-3-methylphenol has been synthesized and found to be identical with the isopropyl-3-methylphenol of m.p. 69 °C. obtained from the isopropylation of m-cresol. 6-Isopropyl-3-methoxytoluene has been synthesized and found to be identical with the methyl ether of the isopropyl-3-methylphenol of m.p. 112 °C., also obtained from the isopropylation of m-cresol.
-
PROCESS AND INTERMEDIATES FOR PREPARING INTEGRASE INHIBITORS申请人:Dowdy Eric公开号:US20130116437A1公开(公告)日:2013-05-09The invention provides synthetic processes and synthetic intermediates that can be used to prepare 4-oxoquinolone compounds having useful integrase inhibiting properties.本发明提供一种合成过程和合成中间体,可用于制备具有有用的整合酶抑制性能的4-氧喹诺酮化合物。
-
ANTHRACENE DERIVATIVE AND ORGANIC ELECTROLUMINESCENT ELEMENT USING THE SAME申请人:Kawamura Masahiro公开号:US20120138914A1公开(公告)日:2012-06-07An anthracene derivative represented by the following formula (1): In the formula (1), Z is a structure represented by the following formula (2). In the formula (2), at least one pair of adjacent two substituents of R 11 to R 18 form a ring represented by the following formula (3) or (4):
表征谱图
-
氢谱1HNMR
-
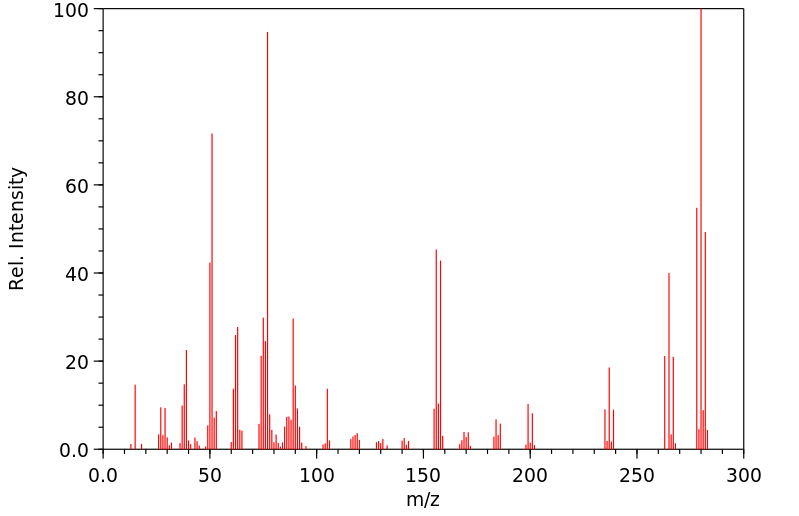
质谱MS
-
碳谱13CNMR
-
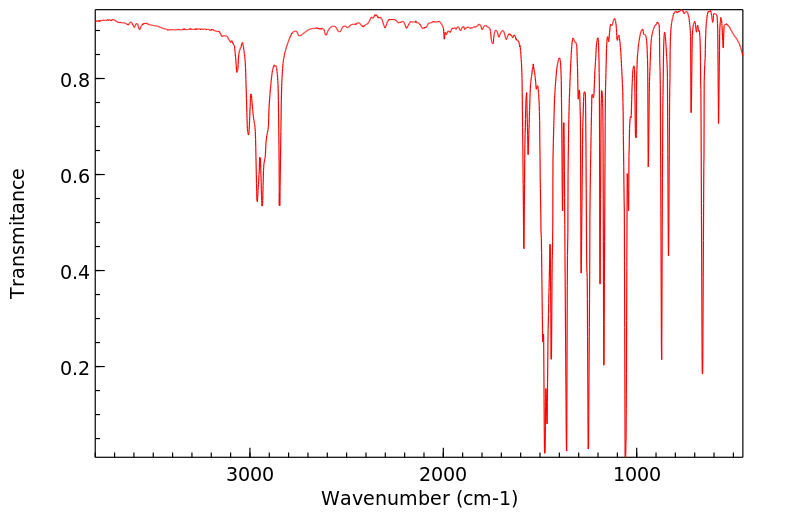
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯