7-乙基-3,4-二氢-2H-1-萘酮 | 22531-06-2
中文名称
7-乙基-3,4-二氢-2H-1-萘酮
中文别名
——
英文名称
7-ethyl-1-tetralone
英文别名
7-ethyltetralone;7-ethyl-3,4-dihydronaphthalen-1(2H)-one;7-Aethyl-tetralon-(1);7-ethyl-3,4-dihydro-2H-naphthalen-1-one
CAS
22531-06-2
化学式
C12H14O
mdl
——
分子量
174.243
InChiKey
BMHUHOFPKFAGAR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:265.23°C (rough estimate)
-
密度:1.0556
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.42
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-bromo-7-ethyl-1,2,3,4-tetrahydronaphthalin-1-one 135517-59-8 C12H13BrO 253.139 —— 5-bromo-7-ethyl-1-tetralone 676136-28-0 C12H13BrO 253.139 6-乙基-1,2,3,4-四氢萘 6-ethyltetralin 22531-20-0 C12H16 160.259
反应信息
-
作为反应物:描述:7-乙基-3,4-二氢-2H-1-萘酮 在 乙酸铵 、 氢氧化钾 、 溶剂黄146 作用下, 以 苯 为溶剂, 反应 45.0h, 生成 1-carboxy-7-ethyltetralin-1-acetic acid参考文献:名称:合成的取代螺化合物催化脱氢过程中的分子重排摘要:7'-甲基-1-羟基螺[tetralin-2,1'-tetralin] 5和7'-乙基-7-甲基-1-羟基螺[tetralin-2,1'-tetralin] 5a的催化脱氢得到3-甲基丙烯7和3-乙基-9-甲基丙烯7a。酸酐的缩合1酸酐与苯和1-羧基-7- methyltetralin -1-乙酸1A的1-羧基-7- ethyltetralin -1-乙酸与甲苯,得到相应的酮-酸2和2a中,其上催化还原得到丁酸3和3a。酸环化(3和3a)提供了螺酮4和4a。用LAH还原螺酮(4和4a)得到螺碳化合物5和5a。DOI:10.1016/s0040-4020(01)88340-x
-
作为产物:描述:3-(4-乙基苯甲酰)丙酸 在 盐酸 、 草酰氯 、 水 、 mercury(II) chloride 、 锌 作用下, 以 二氯甲烷 、 N,N-二甲基甲酰胺 、 甲苯 为溶剂, 反应 37.5h, 生成 7-乙基-3,4-二氢-2H-1-萘酮参考文献:名称:吡咯类作为代孕代苯酚对巨噬细胞迁移抑制因子的强效抑制剂的优化摘要:巨噬细胞迁移抑制因子(MIF)是促炎细胞因子,与炎症,细胞增殖和神经系统疾病的调节有关。MIF也是一种酶,起酮-烯醇互变异构酶的作用。大多数有效的MIF互变异构酶抑制剂都含有苯酚,该苯酚在活性位点与Asn97氢键结合。从113-μ起始米对接命中,我们报告,已经提供了取代的吡唑为具有60-70Ñ效力苯酚替代基于结构的和计算机辅助设计的结果米。MIF与吡唑配合物的晶体结构突出了与Lys32和Asn97的氢键作用,以及与Tyr36,Tyr95和Phe113的芳基-芳基相互作用对结合的贡献。DOI:10.1002/cmdc.201800158
文献信息
-
[EN] PYRAZOLE-CONTAINING MACROPHAGE MIGRATION INHIBITORY FACTOR INHIBITORS<br/>[FR] INHIBITEURS DU FACTEUR INHIBITEUR DE MIGRATION DES MACROPHAGES CONTENANT DU PYRAZOLE申请人:UNIV YALE公开号:WO2019178480A1公开(公告)日:2019-09-19In one aspect, the invention comprises compounds that bind and inhibit macrophage migration inhibitory factor. In another aspect, the invention provides methods of treating inflammatory disease, neurological disorders and cancer using the compounds of the invention.在一个方面,该发明涉及结合并抑制巨噬细胞迁移抑制因子的化合物。在另一个方面,该发明提供使用该发明的化合物治疗炎症性疾病、神经系统疾病和癌症的方法。
-
Aryl(alkyl)propylamides申请人:Adir Et Compagnie公开号:US05731352A1公开(公告)日:1998-03-24The invention relates to a compound selected from those of formula (I): ##STR1## in which A, R.sub.1, R'.sub.1, R.sub.2, R.sub.3 and n are as defined in the description, and medicinal product containing the same useful for treating a mammal afflicted with a disorder of the melatoninergic system.该发明涉及选择自式(I)的化合物:##STR1##其中A、R.sub.1、R'.sub.1、R.sub.2、R.sub.3和n如描述中所定义,并且含有相同化合物的药物制剂,用于治疗患有褪黑激素系统紊乱的哺乳动物。
-
Tetrahydro-1H-benzazepinones and hexahydroazepinones as selective申请人:Pfizer Inc.公开号:US05618811A1公开(公告)日:1997-04-08This invention relates to compounds of formula (I) wherein R.sup.1, R.sup.2, R.sup.3, R.sup.4 and X are as defined in the application. These compounds are CCK-B receptor antagonists and are useful in the treatment and prevention of central nervous system and gastrointestinal disorders. ##STR1##这项发明涉及式(I)的化合物,其中R.sup.1、R.sup.2、R.sup.3、R.sup.4和X如申请中所定义。这些化合物是CCK-B受体拮抗剂,可用于治疗和预防中枢神经系统和胃肠道疾病。
-
5-Phenyl-3-ureidobenzazepin-2-ones as Cholecystokinin-B Receptor Antagonists作者:John A. Lowe、David L. Hageman、Susan E. Drozda、Stafford McLean、Dianne K. Bryce、Rosemary T. Crawford、Stevin Zorn、Jean Morrone、Jon BordnerDOI:10.1021/jm00048a015日期:1994.10A series of 5-phenyl-3-ureidobenzazepin-2-one cholecystokinin-B (CCK-B) receptor antagonists was synthesized using Beckmann ring expansion of a suitable 4-phenyl-1-tetralone as a key step. Structure-activity relationship studies revealed the importance of the 5-phenyl group for potent and selective CCK-B affinity. Addition of an 8-methyl substituent and resolution provided the potent (CCK-B IC50 =
-
A simple procedure for the synthesis of 3-(substituted-sulfanyl)-4-hydroxy-6-substituted-pyran-2-ones作者:K. S. Para、E. L. Ellsworth、J. V. N. Vara PrasadDOI:10.1002/jhet.5570310657日期:1994.11A series of 3-(substituted sulfanyl)-4-hydroxy-6-substituted-pyran-2-ones were synthesized for Human immunodeficiency virus-1 protease inhibition. These compounds were synthesized in a simple and convergent fashion to allow us a rapid preparation of many structurally diversified analogues. Thus the condensation of trimethylsilyl enol ethers of corresponding ketones, with 2-(S-substituted)propane-1
表征谱图
-
氢谱1HNMR
-
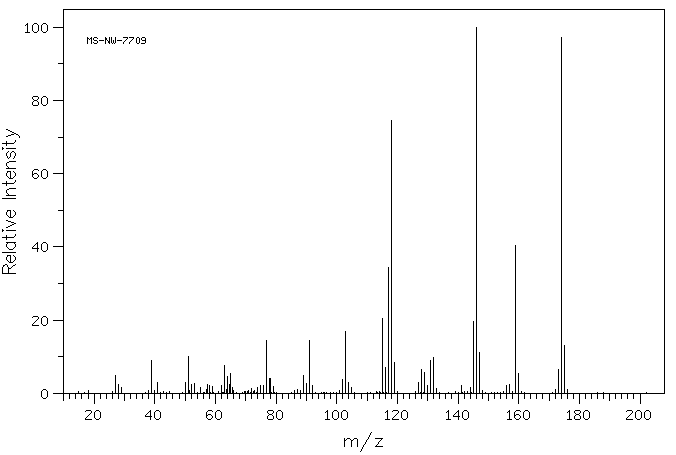
质谱MS
-
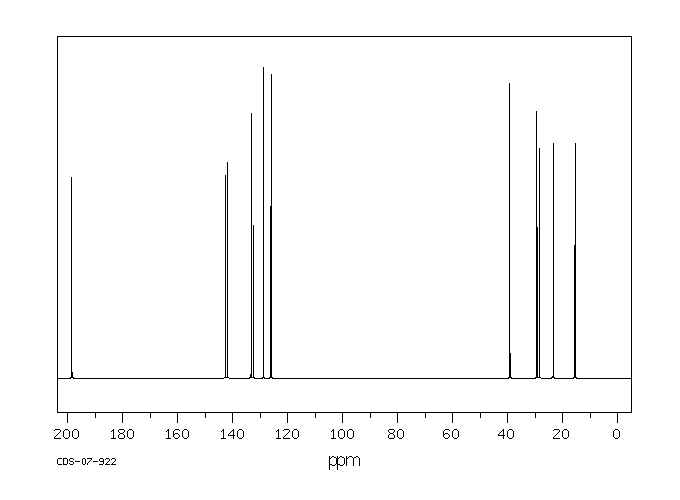
碳谱13CNMR
-
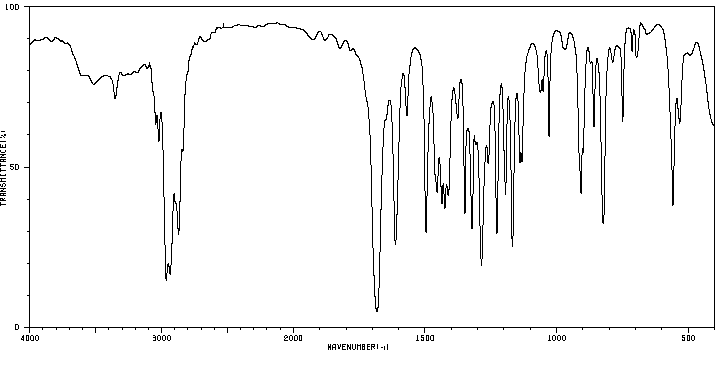
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-(+)-5,5'',6,6'',7,7'',8,8''-八氢-3,3''-二叔丁基-1,1''-二-2-萘酚,双钾盐
顺式-4-(4-氯苯基)-1,2,3,4-四氢-N-甲基-1-萘胺盐酸盐
顺式-4-(3,4-二氯苯基)-1,2,3,4-四氢N-叔丁氧羰基-1-萘胺
顺式-1-苯甲酰氧基-2-二甲基氨基-1,2,3,4-四氢萘
顺式-1,2,3,4-四氢-5-环氧丙氧基-2,3-萘二醇
顺式-(1S,4S)-N-甲基-4-(3,4-二氯苯基)-1,2,3,4-四氢-1-萘胺扁桃酸盐
顺-5,6,7,8-四氢-6,7-二羟基-1-萘酚
顺-(+)-5-甲氧基-1-甲基-2-(二正丙基氨基)萘满马来酸
阿洛米酮
阿戈美拉汀杂质醇(A)
阿戈美拉汀杂质
钠2-羟基-7-甲氧基-1,2,3,4-四氢-2-萘磺酸酯
金钟醇
邻烯丙基苯基溴化镁
那高利特盐酸盐
那高利特
过氧化,1,1-二甲基乙基1,2,3,4-四氢-1-萘基
贝多拉君
螺<4.7>十二烷
蔡醇酮
萘磺酸,二癸基-1,2,3,4-四氢-
萘并[2,3-d]噁唑-2,5-二酮,3,6,7,8-四氢-3-甲基-
萘并[2,3-d]咪唑,2-乙基-5,6,7,8-四氢-(6CI)
萘亚胺
苯甲酸-(5,6,7,8-四氢-[2]萘基酯)
苯甲丁氮酮
苯甲丁氮酮
苯甲丁氮酮
苯并烯氟菌唑
苄基[(2S)-7-羟基-1,2,3,4-四氢萘-2-基]氨基甲酸酯
苄基-5-甲氧基-1,2,3,4-四氢萘-2-基氨基甲酸酯
苄基(1,2,3,4-四氢萘-2-基)胺
舍曲林二甲基杂质盐酸盐
舍曲林EP杂质B
舍曲林2,3-二氯亚胺杂质
舍曲林
羟甲基四氢萘酚
羟基-苯基-(5,6,7,8-四氢-[2]萘基)-乙酸
美曲唑啉
罗替戈汀硫酸盐
罗替戈汀杂质19
罗替戈汀杂质18
罗替戈汀杂质11
罗替戈汀中间体
罗替戈汀中间体
罗替戈汀
罗替戈汀
纳多洛尔杂质
米贝地尔(二盐酸盐)
硅烷,[3-(3,4-二氢-1(2H)-萘亚基)-1-炔丙基]三甲基-,(Z)-