2,3-吡嗪二甲酰胺 | 6164-78-9
中文名称
2,3-吡嗪二甲酰胺
中文别名
——
英文名称
pyrazine-2,3-dicarboxamide
英文别名
Pyrazin-2,3-dicarbonsaeure-diamid;NSC 93820
CAS
6164-78-9
化学式
C6H6N4O2
mdl
MFCD00006133
分子量
166.139
InChiKey
TZMYZOQDDVSLJU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:248 °C (dec.) (lit.)
-
沸点:294.33°C (rough estimate)
-
密度:1.4434 (rough estimate)
-
稳定性/保质期:
常温常压下稳定,避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):-2
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:112
-
氢给体数:2
-
氢受体数:4
安全信息
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:2934999090
SDS
| Name: | 2 3-Pyrazinedicarboxamide 97% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 6164-78-9 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 6164-78-9 | 2,3-Pyrazinedicarboxamide | 97% | 228-197-0 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 6164-78-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 248 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C6H6N4O2
Molecular Weight: 166.14
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Acids, bases, oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 6164-78-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,3-Pyrazinedicarboxamide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 6164-78-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 6164-78-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 6164-78-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-氰基-2-吡嗪甲酰胺 3-cyanopyrazine-2-carboxamide 66505-29-1 C6H4N4O 148.124 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-氰基-2-吡嗪甲酰胺 3-cyanopyrazine-2-carboxamide 66505-29-1 C6H4N4O 148.124 2,3-吡嗪二羧酰胺 pyrazine-2,3-dicarboximide 4933-19-1 C6H3N3O2 149.109 吡嗪-2,3-二羧酸单酰胺 pyrazine-2,3-dicarboxylic acid monoamide 67367-37-7 C6H5N3O3 167.124
反应信息
-
作为反应物:描述:参考文献:名称:Azaisatoic anhydrides摘要:一种从相应的酸、二羧酰胺、2,3-和3,4-吡啶二羧酰胺以及N-单取代的2,3-和3,4-吡啶二羧酰胺中制备杂环酸酐和嘧啶二酮的方法,其中上述化合物在适当无水惰性溶剂的存在下与醋酸四铅反应。公开号:US03947416A1
-
作为产物:描述:参考文献:名称:Gabriel; Sonn, Chemische Berichte, 1907, vol. 40, p. 4852摘要:DOI:
文献信息
-
Immunosuppressive effects of pteridine derivatives申请人:——公开号:US20030236255A1公开(公告)日:2003-12-25Novel poly-substituted pteridinediones (lumazines), and mono- or polysubstituted 2-thiolumazines, 4-thiolumazines or 2,4-dithiolumazines, having disclosed substituents in positions 1, 3, 6 and 7 of the pteridine ring, and pharmaceutically acceptable salts thereof, are useful as biologically active ingredients in preparing pharmaceutical compositions especially for the treatment or prevention of a CNS disorder, a cell proliferative disorder, a viral infection, an immune or auto-immune disorder or a transplant rejection. Combinations of the pteridine derivatives of the invention with an immunosuppressant or immunomodulator drug, an antineoplastic drug or an antiviral agent, providing potential synergistic effects, are also disclosed.
-
Dipeptidyl peptidase-IV inhibitors申请人:Hulin Bernard公开号:US20050234065A1公开(公告)日:2005-10-20The invention provides compounds of Formula (I) or prodrugs thereof, or pharmaceutically acceptable salts of said compounds or prodrugs, or solvates of said compounds, prodrugs or salts, wherein A, N, X and R 1 are as defined herein; pharmaceutical compositions thereof; and methods of using the pharmaceutical compositions for the treatment of diseases, including Type 2 diabetes, Type 1 diabetes, impaired glucose tolerance, hyperglycemia, metabolic syndrome (syndrome X and/or insulin resistance syndrome), glucosuria, metabolic acidosis, arthritis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, short stature due to growth hormone deficiency, infertility due to polycystic ovary syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome; short bowel syndrome; and the prevention of disease progression in Type 2 diabetes.该发明提供了Formula (I)的化合物或其前药,或者该化合物或前药的药用可接受盐,或者该化合物、前药或盐的溶剂化合物,其中A、N、X和R1如本文所定义;以及这些药物组合物;以及使用这些药物组合物治疗疾病的方法,包括2型糖尿病、1型糖尿病、糖耐量受损、高血糖、代谢综合征(综合征X和/或胰岛素抵抗综合征)、葡萄糖尿病、代谢性酸中毒、关节炎、白内障、糖尿病神经病、糖尿病肾病、糖尿病视网膜病、糖尿病心肌病、肥胖、由肥胖恶化的疾病、高血压、高脂血症、动脉粥样硬化、骨质疏松症、脆弱、骨质流失、骨折、急性冠状动脉综合征、生长激素缺乏引起的矮小症、多囊卵巢综合征引起的不孕症、焦虑、抑郁、失眠、慢性疲劳、癫痫、进食障碍、慢性疼痛、酒精成瘾、与肠道蠕动相关的疾病、溃疡、肠易激综合征、炎症性肠病;短肠综合征;以及预防2型糖尿病疾病进展。
-
Magnetic properties and molecular structure of copper(II) complexes of 2,3-pyrazinedicarboxamide作者:Cheryl L. Klein、Edwin D. Stevens、Charles J. O'Connor、Richard J. Majeste、Louis M. TrefonasDOI:10.1016/s0020-1693(00)82795-4日期:1983.1The synthesis, crystal structures, and magnetic properties of several copper(II) complexes of 2,3-pyrazinedicarboxamide [L = C 4 H 2 N 2 (CONH 2 ) 2 ] are reported. The crystal structures of CuL 2 (OH) 2 · 2H 2 O and [CuLCl 2 ] 2 were determined from single crystal x-ray diffraction using counter methods, and the magnetic susceptibility of these complexes plus the complex CuL 2 (ClO 4 ) 2 were studied摘要报道了2,3-吡嗪二羧酰胺[L = C 4 H 2 N 2(CONH 2)2]的几种铜(II)配合物的合成,晶体结构和磁性。使用对立法通过单晶X射线衍射确定CuL 2(OH)2·2H 2 O和[CuLCl 2] 2的晶体结构,以及这些配合物与配合物CuL 2(ClO 4)2的磁化率。在6–300 K的温度范围内进行了研究。CuL 2(OH)2·2H 2 O结晶为单体配合物,在未配位的水分子与吡嗪羧酰胺基团之间具有氢键键合。[CuLCl 2] 2结晶为具有反转中心的二聚体复合物。CuLCl 2配位球是正方形平面。二聚体是由两个桥连的氯形成五配位的铜(II)单元而形成的。CuL 2(OH)2·2H 2 O和CuL 2(H 2 O)2的磁性与简单居里定律一致。氯桥二聚体[CuLCl 2] 2的磁化率表现出反铁磁耦合(g = 2.11,J = -11.5cm -1)。CuL 2(OH)2·2H
-
The Coordination of the Perchlorate Ion. I. The Crystal and Molecular Structure of Bis(perchlorato)bis(pyrazine-2,3-dicarboxamide)copper(II)作者:Masao SekizakiDOI:10.1246/bcsj.54.146日期:1981.1Bis(perchlorato)bis(pyrazine-2,3-dicarboxamide)copper(II) has been prepared, and its crystal and molecular structure has been determined by the X-ray diffraction and the infrared spectroscopic methods. The crystals are triclinic with a space group P\bar1; a=10.959(4), b=6.970(3), c=7.666(3) A, α=116.63(3), β=78.14(5), and γ=97.27(5)°, and Z=1. The refinement of the crystal structure has been carried制备了双(高氯根)双(吡嗪-2,3-二甲酰胺)铜(II),并通过X射线衍射和红外光谱法确定了其晶体和分子结构。晶体为三斜晶系,空间群为 P\bar1;a=10.959(4),b=6.970(3),c=7.666(3) A,α=116.63(3),β=78.14(5),γ=97.27(5)°,Z=1。晶体结构的细化是通过块对角最小二乘法进行的,对于 1727 次非零反射给出 R=0.054。该配合物是中心对称的,两个双齿配体分子通过酰胺氧和吡嗪氮原子与反位的铜原子螯合,形成一个方形平面。两个高氯酸根离子通过该平面顶部和底部的氧原子与铜原子弱配位;这样,就形成了一个细长的八面体。复杂的分子通过 N-O 型氢键相互连接,形成三维网络。红外光谱已与 t...
-
Preparation, structural characterisation, and thermal and electrical studies of complexes of cobalt, nickel and zinc with 2,3-pyrazinedicarboxamide作者:J.R. Allan、A.D. Paton、K. TurveyDOI:10.1016/0040-6031(91)87046-y日期:1991.9with cobalt, nickel and zinc were prepared and isolated from aqueous solution. Spectral and magnetic studies showed that the cobalt and nickel compounds have octahedral structures while the zinc compound has a tetrahedral structure. The thermal behaviour of these compounds was studied by thermogravimetry and differential thermal analysis. The compounds are hydrated and they decompose thermally by losing摘要 从水溶液中制备并分离出2,3-吡嗪二甲酰胺与钴、镍和锌的化合物。光谱和磁性研究表明,钴和镍化合物具有八面体结构,而锌化合物具有四面体结构。通过热重分析和差热分析研究了这些化合物的热行为。这些化合物被水合,它们通过失水热分解,然后是有机配体,得到金属氧化物。对于所制备的化合物,室温电导率测量给出8.2×10 -8 Ω -1 m -1 至1.3×10 -7 Ω -1 m -1 范围内的结果。结构和电性能之间的相关性很明显,因为金属环境是八面体的两种配合物(即 钴和镍配合物)表现出锌配合物(其中金属环境为四面体)未观察到的极性效应。其效果是在反转施加的极性时电导率增加,但在恢复初始极性时增加的电导率保持不变。获得了每种化合物的电导率的温度依赖性,并用于推断传导的近似活化能。
表征谱图
-
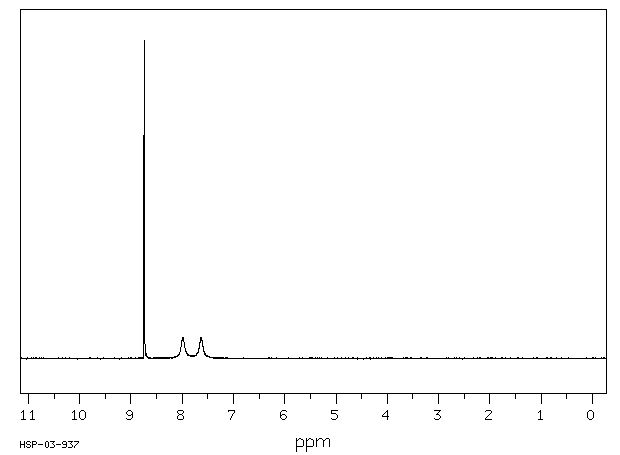
氢谱1HNMR
-
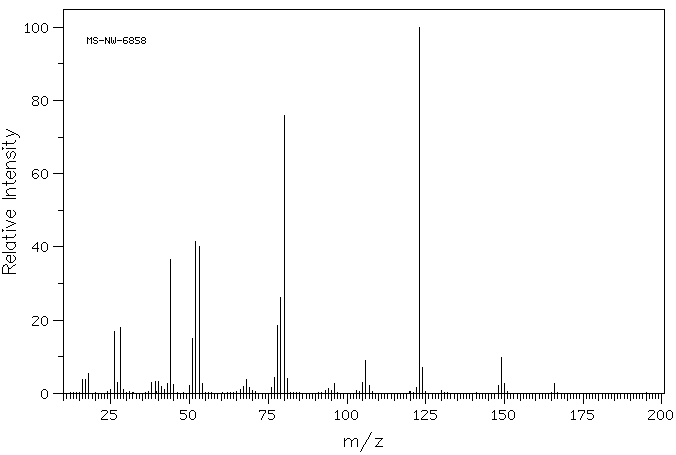
质谱MS
-
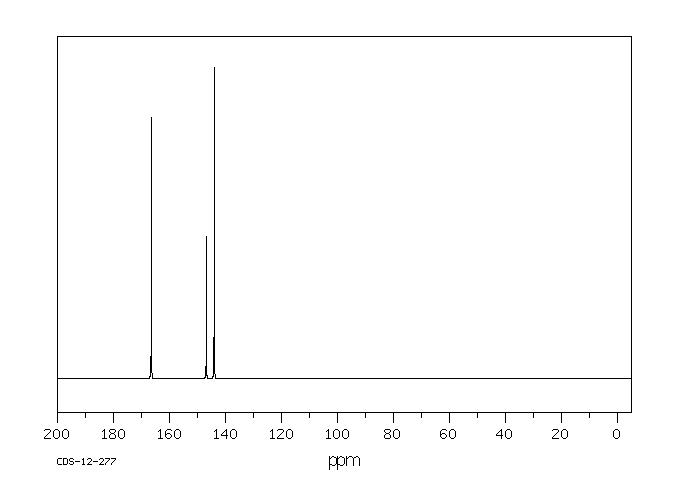
碳谱13CNMR
-
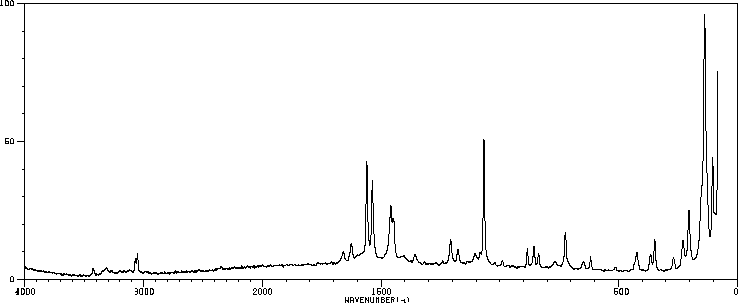
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3