香豆素 2 | 26078-25-1
中文名称
香豆素 2
中文别名
香豆素2;7-(乙氨基)-4,6-二甲基香豆素;香豆素450;4,6-二甲基-7-乙基氨基香豆素;7-(乙氨基)-4,6-二甲基-2H-1-苯并吡喃-2-酮
英文名称
coumarin 2
英文别名
4,6-dimethyl-7-(ethylamino)coumarin;7-ethyl-amino-4,6-dimethyl coumarin;4,6-dimethyl-7-ethylaminocoumarin;7-ethylamino-4,6-dimethylcoumarin;coumarin 450;coumarin-450;7-(ethylamino)-4,6-dimethylchromen-2-one
CAS
26078-25-1
化学式
C13H15NO2
mdl
MFCD00006860
分子量
217.268
InChiKey
QZXAEJGHNXJTSE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:168-170 °C
-
沸点:357.82°C (rough estimate)
-
密度:1.0960 (rough estimate)
-
最大波长(λmax):366nm(MeOH)(lit.)
-
稳定性/保质期:
遵照规定使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:16
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.307
-
拓扑面积:38.3
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xn
-
安全说明:S26,S37/39
-
危险类别码:R20/21/22,R36/37/38
-
海关编码:2932209090
-
WGK Germany:-
-
危险性防范说明:P264,P280,P302+P352,P337+P313,P305+P351+P338,P362+P364,P332+P313
-
危险性描述:H315,H319
SDS
| Name: | Coumarin 2 Laser Grade 99+% (UV-Vis) Material Safety Data Sheet |
| Synonym: | 4,6-Dimethyl-7-ethylaminocoumarin ; 2H-1-Benzopyran-2-One,7-(Ethylamino)-4,6-Dimethyl- |
| CAS: | 26078-25-1 |
Synonym:4,6-Dimethyl-7-ethylaminocoumarin ; 2H-1-Benzopyran-2-One,7-(Ethylamino)-4,6-Dimethyl-
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 26078-25-1 | 2H-1-Benzopyran-2-One,7-(Ethylamino)-4 | >99 | 247-446-4 |
Risk Phrases: 20/21/22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
The toxicological properties of this substance have not been fully investigated.
Inhalation:
Harmful if inhaled. Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 26078-25-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: yellow to yellow-green
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 168 - 170 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: slightly soluble in water
Specific Gravity/Density:
Molecular Formula: C13H15NO2
Molecular Weight: 217.27
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong acids, strong bases.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 26078-25-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2H-1-Benzopyran-2-One,7-(Ethylamino)-4,6-Dimethyl- - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
RID/ADR
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 37/39 Wear suitable gloves and eye/face
protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 26078-25-1: No information available.
Canada
CAS# 26078-25-1 is listed on Canada's DSL List.
CAS# 26078-25-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 26078-25-1 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-{[(4,6-Dimethyl-2-oxo-2H-chromen-7-yl)-ethyl-amino]-methyl}-benzoic acid 575490-98-1 C21H21NO4 351.402 —— 3,5-Bis(N-(4,6-dimethyl-7-ethylaminocoumarin)methyl)phenol 234096-68-5 C34H36N2O5 552.67 —— 3,5-bis(N-(4,6-dimethyl-7-ethylaminocoumarin)methyl)anisole 249630-29-3 C35H38N2O5 566.697 —— [3,5-Bis[[(4,6-dimethyl-2-oxochromen-7-yl)-ethylamino]methyl]phenyl] hexadecane-1-sulfonate 249630-35-1 C50H68N2O7S 841.165 —— [3,5-Bis[[3,5-bis[[(4,6-dimethyl-2-oxochromen-7-yl)-ethylamino]methyl]phenoxy]methyl]phenyl] hexadecane-1-sulfonate 234096-73-2 C92H110N4O13S 1511.97
反应信息
-
作为反应物:描述:香豆素 2 在 sodium hydroxide 、 potassium carbonate 作用下, 以 乙醇 、 乙腈 为溶剂, 反应 75.0h, 生成 3,5-Bis(N-(4,6-dimethyl-7-ethylaminocoumarin)methyl)phenol参考文献:名称:新型聚合构造树枝状聚合物的光收集和能量转移。摘要:通过将相互作用的激光染料附着到树枝状大分子的链端和焦点,可以有效地将树枝状大分子的大外围天线(参见左图)收集的能量直接漏到中央荧光芯上(图左)在中间)的过程中不涉及树枝状大分子内部骨架。然后,能量从纤芯以窄带荧光辐射的形式发出(右图)。DOI:10.1002/(sici)1521-3773(19990517)38:10<1422::aid-anie1422>3.0.co;2-v
-
作为产物:描述:3-(乙基氨基)-4-甲酚 、 乙酰乙酸乙酯 以38%的产率得到参考文献:名称:GRANDBERG, I. I.;DENISOV, L. K.;MELNIKOVA, L. M.;POPOVA, O. A.;TOKMAKOV, +, IZV. TIMIRYAZEV. S.-X. AKAD., 1984, N 4, 149-152摘要:DOI:
文献信息
-
[EN] COMPOUNDS HAVING ELECTROLUMINESCENT OR ELECTRON TRANSPORT PROPERTIES<br/>[FR] COMPOSÉS PRÉSENTANT DES PROPRIÉTÉS ÉLECTROLUMINESCENTES OU DE TRANSPORT DES ÉLECTRONS申请人:MERCK PATENT GMBH公开号:WO2009112854A1公开(公告)日:2009-09-17A compound of the formula R1 (CR3 =CR4 ) nAr(CR4 =CR3 ) nR2 wherein: n is 0 or 1; Ar represents aryl or heteroaryl having 1-5 aromatic rings which may be chain or fused or a combination of chain and fused, which may be substituted withalkoxy, fluoro, fluoroalkyl or cyano and which in the case of a 5-memnered ring nitrogen heteroatom may be N-substituted with aryl or substituted aryl optionally further substituted with alkoxy, fluoro, fluoroalkyl or cyano; R1 and R2 independently represent aryl or nitrogen, oxygen or sulphur-containing heteroaryl having two to four fused aromatic rings one of which may be 5-membered and optionally substituted by aryl or heteroaryl having 1-5 chain or fused aromatic rings which may be further substituted withalkoxy, fluoro, fluoroalkyl or cyano; and R3 and R4 independently represent hydrogen, methyl, ethyl or benzyl. Alsoprovided is a method of making a compound having electroluminescent and/or electroconductive properties, which comprises condensing an aromatic dialdehyde with a methyl-substituted heteroaryl compound having two to four fused rings which maybe unsubstituted ormay be further substituted by aryl or heteroaryl having from one to fouraromatic rings, said aryl or heteroaryl substituent or substituents optionally being substituted with one or more fluoro, fluoroalkyl orcyano substituents.The condensation may be carried out in acetic anhydride under reflux. The compounds may be incorporatede.g. as an electron transport layer into an optical light emitting diode,where embodiments can provide high electron mobility, low turn-on voltage and good lifetime,or into an electrostatic imaging member. Acompositionis provided comprising a compound as above and a second host or electron transport material and/or at leastone dopant.一个化合物的结构式为R1(CR3=CR4)nAr(CR4=CR3)nR2,其中:n为0或1;Ar代表含有1-5个芳香环的芳基或杂芳基,可以是链状的、融合的或链状和融合的组合,可以被烷氧基、氟、氟烷基或氰基取代,在5-成员环氮杂原子的情况下,可能被芳基或取代芳基N-取代,可选择地进一步取代为烷氧基、氟、氟烷基或氰基;R1和R2独立地代表含有两到四个融合芳香环的芳基或氮、氧或硫杂杂芳基,其中一个可能是5-成员的,并且可选择地被芳基或含有1-5个链状或融合芳香环的杂芳基取代,这些芳基或杂芳基可能进一步被烷氧基、氟、氟烷基或氰基取代;R3和R4独立地代表氢、甲基、乙基或苄基。还提供了一种制备具有电致发光和/或电导性能的化合物的方法,包括将芳香二醛与一种具有两到四个融合环的甲基取代的杂芳基化合物缩合,该化合物可能未取代或可能进一步被含有一到四个芳香环的芳基或杂芳基取代,所述芳基或杂芳基取代物可选择地被一个或多个氟、氟烷基或氰基取代。缩合可以在乙酸酐中回流进行。这些化合物可以被用作电子传输层,例如用于光电发光二极管,其中实施例可以提供高电子迁移率、低开启电压和良好的寿命,或用于静电成像成员。提供了一种组合物,包括上述化合物和第二宿主或电子传输材料和/或至少一个掺杂剂。
-
Copper-catalyzed synthesis of α-amino nitriles through methyl transfer from DMF to aromatic amines作者:Zaifeng Yuan、Na Li、Chunyu Zhu、Chengfeng XiaDOI:10.1039/c8cc00485d日期:——A copper-catalyzed activation of C(sp3)–H bonds of DMF at room temperature was developed, which results in methyl transfer to aromatic amines for efficient synthesis of exceedingly valuable α-amino nitriles. This process features excellent functional group tolerance, a broad substrate scope, and high activity under ambient conditions.
-
Copper-catalyzed amination of an α-C(sp<sup>3</sup>)–H bond in inactivated ethers to synthesize α-aminonitriles作者:Zaifeng Yuan、Chunyu Zhu、Zhixian Ma、Chengfeng XiaDOI:10.1039/c8cc06111d日期:——copper-catalyzed functionalization of inert cyclic ethers was developed to provide α-aminonitriles via a cascade oxidation/amination/ring-opening/cyanation reaction. A series of highly versatile α-aminonitriles were obtained from primary or secondary anilines, and heterocyclic and aliphatic amines with high yields. This process features excellent functional group tolerance, a broad substrate scope, and high
-
Synthesis of asymmetrically substituted cyclen-based ligands for the controlled sensitisation of lanthanides作者:K. Eszter Borbas、James I. BruceDOI:10.1039/b705757a日期:——A series of unsymmetrical cyclen-based ligands incorporating an antenna and a quencher have been prepared for the complexation of the visible- (Eu, Tb) and near IR-emitting (Nd, Yb) lanthanides. Eu and Tb were sensitised with coumarin 2, and Nd and Yb with rhodamine. Both antennae were paired with nucleoside (uridine and adenosine) quenchers. The interaction between the quencher and the antenna can be regulated by the addition of the complementary base or DNA to the complexes, resulting in changes in the lanthanide luminescence intensity and lifetime.
-
A FRET approach to phosgene detection作者:Hexiang Zhang、Dmitry M. RudkevichDOI:10.1039/b614725a日期:——A FRET approach towards potential detection of phosgene is presented, which is based on a selective chemical reaction between phosgene (or triphosgene as a simulant) and donor and acceptor fluorophores.
表征谱图
-
氢谱1HNMR
-
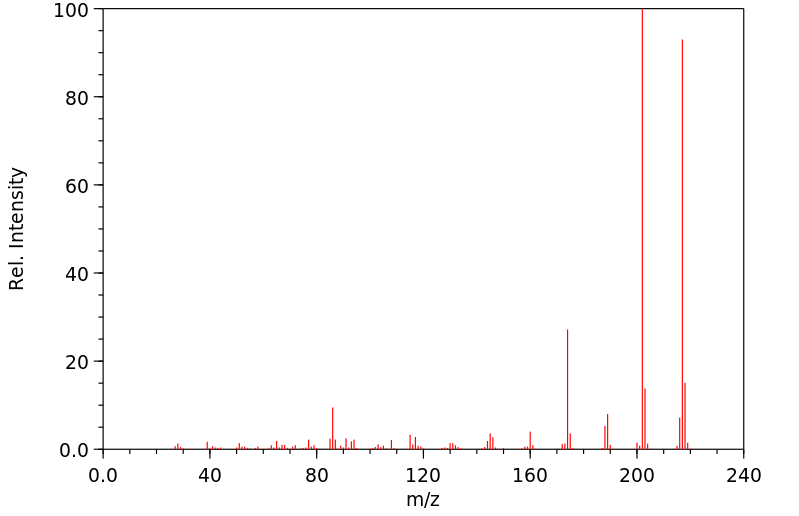
质谱MS
-
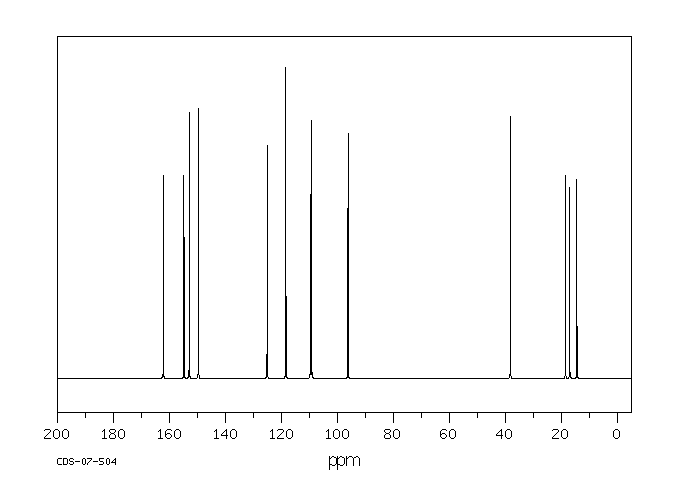
碳谱13CNMR
-
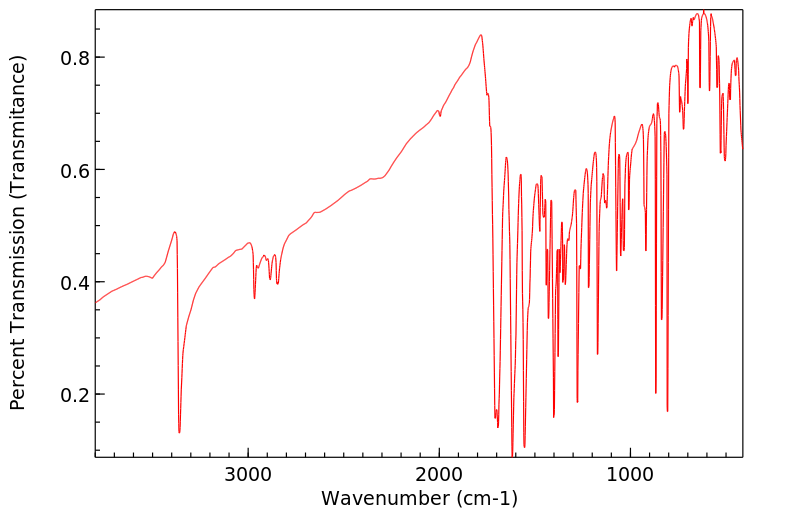
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄皮香豆精
黄木亭
黄曲霉素P2
黄曲霉素P1
黄曲霉素G2-13C17-同位素
黄曲霉素G2
黄曲霉素G1-13C17-同位素
黄曲霉素B2-13C17-同位素
黄曲霉素B1-13C17-同位素
黄曲霉素B1 8,9-环氧化物
黄曲霉素 G1
黄曲霉毒醇Ⅱ
黄曲霉毒醇M1
黄曲霉毒醇A
黄曲霉毒素M2
黄曲霉毒素M1-(O-羧甲基)肟
黄曲霉毒素G2a
黄曲霉毒素G19,10-环氧化物
黄曲霉毒素B2
黄曲霉毒素B1二氯化物
黄曲霉毒素B1-8,9-二氯化物
黄曲霉毒素B1-(O-羧甲基)肟
黄曲霉毒素 Q1
黄曲霉毒素 M1
黄曲霉毒素 B2
黄曲霉毒素 B1
黄曲霉毒素
香豆霉素
香豆素6H
香豆素545T
香豆素545
香豆素525
香豆素343甲酯
香豆素338
香豆素314T
香豆素175
香豆素152
香豆素106
香豆素-D4
香豆素-6-磺酰氯
香豆素-6-甲醛
香豆素-5-氧丁酸
香豆素-4-乙酸
香豆素-3腈
香豆素-35
香豆素-3-羧酸酸酐
香豆素-3-羧酸琥珀酰亚胺酯
香豆素-3-羧酸乙酯
香豆素-3-羧酸
香豆素-3-甲酰氯