3a,4,5,6-四氢琥珀酰亚胺并(3,4-b)-苊-10-酮 | 4756-92-7
中文名称
3a,4,5,6-四氢琥珀酰亚胺并(3,4-b)-苊-10-酮
中文别名
3A,4,5,6-四氢琥珀酰亚胺基[3,4-B]苊-10-酮
英文名称
3a,4,5,6-tetrahydrosuccinimido<3,4-b>acenaphthen-10-one
英文别名
3a,4,5,6-tetrahydrosuccinimido[3,4-b]acenapthen-10-one;5,6-dihydro-4H-acenaphtho[1,8a-c]pyrrole-1,3,10-trione;3a,4,5,6-tetrahydrosuccinimido[3,4-b]acenaphthen-10-one;5,6-Dihydro-1H,4H-acenaphtho(1,8a-c)pyrrole-1,3,10(2H,10aH)-trione;13-azatetracyclo[7.5.1.01,11.05,15]pentadeca-5(15),6,8-triene-10,12,14-trione
CAS
4756-92-7
化学式
C14H11NO3
mdl
——
分子量
241.246
InChiKey
KWUXACIWMYKRCI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:250-254 °C(lit.)
-
沸点:538.4±50.0 °C(Predicted)
-
密度:1.47±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:18
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.36
-
拓扑面积:63.2
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2933990090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-methyl-3a,4,5,6-tetrahydrosuccinimido<3,4-b>acenaphthen-10-one 112531-06-3 C15H13NO3 255.273 —— 2,10a-dimethyl-3a,4,5,6-tetrahydrosuccinimido<3,4-b>acenaphthen-10-one 78052-29-6 C16H15NO3 269.3 —— 2-(4-Nitrophenethyl)-2,3,5,6,10,10a-hexahydro-1H,4H-acenaphtho[1,8a-c]pyrrole-1,3,4-trione 252960-96-6 C22H18N2O5 390.395 —— 5-[2-(1,3,4-Trioxo-2,3,5,6,10,10a-hexahydro-1H,4H-acenaphtho[1,8a-c]pyrrolyl)ethyl]-3-[2-(dimethylamino)ethyl]-1H-indole-2-carboxylic acid 252960-72-8 C29H29N3O5 499.566 —— Ethyl 5-[2-(1,3,4-trioxo-2,3,5,6,10,10a-hexahydro-1H,4H-acenaphtho[1,8a-c]pyrrolyl)ethyl]-3-[2-(dimethylamino)ethyl]-1H-indole-2-carboxylate 252960-70-6 C31H33N3O5 527.62 —— Benzyl 5-[2-(1,3,4-trioxo-2,3,5,6,10,10a-hexahydro-1H,4H-acenaphtho[1,8a-c]pyrrolyl)ethyl]-3-[2-(dimethylamino)ethyl]-1H-indole-2-carboxylate 252960-71-7 C36H35N3O5 589.691 —— 2,3,6,7-tetrahydro-3-oxo-4H-benz isoquinoline-4,4a-(5H)dicarboximide 78052-33-2 C14H12N2O3 256.261 —— 2,3,8,9-tetrahydro-3-oxo-1H-benz isoquinoline-1,9a-(7H)dicarboximide 78052-30-9 C14H12N2O3 256.261
反应信息
-
作为反应物:描述:3a,4,5,6-四氢琥珀酰亚胺并(3,4-b)-苊-10-酮 在 sodium hydride 、 potassium carbonate 作用下, 以 四氢呋喃 、 N,N-二甲基甲酰胺 为溶剂, 反应 5.0h, 生成 2,10a-dimethyl-3a,4,5,6-tetrahydrosuccinimido<3,4-b>acenaphthen-10-one参考文献:名称:Campaigne, E.; Huffman, J. C.; Yodice, Richard, Journal of Heterocyclic Chemistry, 1981, vol. 18, p. 135 - 142摘要:DOI:
文献信息
-
Synthesis and serotonergic activity of a series of 2-(N-benzyl)carboxamido-5-substituted-N,N-dimethyltryptamine derivatives: novel antagonists for the vascular 5-HT1B-like receptors作者:Gerard P. Moloney、Graeme R. Martin、Neil Mathews、Heather Hobbs、Susan Dodsworth、Pang Yih Sang、Cameron Knight、Miles Maxwell、Robert C. GlenDOI:10.1039/a903141c日期:——The synthesis and vascular 5-HT1B-like receptor activity of a novel series of 2-(N-benzyl)carboxamido-5-substituted-N,N-dimethyltryptamine derivatives is described. Modifications to the 5-ethylene linked heterocycle are explored. Compounds such as N-benzyl-5-[2-(phthalimido)ethyl]-3-[2-(dimethylamino)ethyl]-1H-indole-2-carboxamide 22 (pKB = 7.33), the 2-aminobenzyl analogue 24 (pKB = 7.19), which both contain a phthalimide group, and N-benzyl-5-[2-(1-benzyl-2,5-dioxoimidazolidin-4-yl)ethyl]-3-[2-(dimethylamino)ethyl]-1H-indole-2-carboxamide 81 (pKB = 7.05), which incorporates an N-benzylhydantoin moiety, have good 5-HT1B-like affinity and indicate that there may be a hydrophobic binding pocket within the vascular 5-HT1B-like receptor previously not considered. Compounds including N-benzyl-3-[2-(dimethylamino)ethyl]-5-[2-(2,4-dioxo-1,3-thiazolidinyl)ethyl]-1H-indole-2-carboxamide 39 (pKB = 7.35) and the dimethyl analogue 46 (pKB = 7.48) which contain a 2,4-thiazolidinedione moiety have good vascular 5-HT1B-like receptor affinity and show that the sulfur atom is well tolerated. Compound 61 which includes a methylsulfonyl substituent on the 1-nitrogen of the hydantoin ring system has the highest recorded 5-HT1B-like affinity for this series (pKB = 7.54) and it is proposed that this functional group can interact with a secondary hydrogen bonding region within the receptor. Compounds 22, 24, 39, 46, 61 and 81 also exhibited good selectivity over the α1-adrenoceptors. The most selective compound from this series is 46 which contains a 5,5-dimethylthiazolidine-2,4-dione group and which is 66-fold selective over the α1-adrenoceptors. This finding is consistent with the previous discovery that 5,5-dimethyl substitution on the hydantoin group in a related series of compounds afforded superior selectivity for 5-HT1B-like receptors over α1-adrenoceptors and other 5-HT receptors, in particular 5-HT2A receptors, relative to unsubstituted hydantoin analogues. The selectivity of these compounds for the vascular 5-HT1B-like receptor is discussed. Structure–activity relationship indicated a significant steric requirement of the 5-HT1B-like receptor subtype. Potential modes of binding for several of the compounds to a vascular 5-HT1B-like receptor pharmacophore model are also proposed.0)。
-
Cyclic imide-substituted pyridylalkane, alkene, alkine carboxamides useful as cytostatic and immunosuppressive agents申请人:Astellas Pharma GmbH公开号:US07192967B1公开(公告)日:2007-03-20The invention relates to new pyridylalkane, alkene, and alkine carboxamides substituted with an imide with a saturated or one or several-fold unsaturated hydrocarbon residue in the carboxylic acid group according to general formula (I), wherein the residue E is a cyclic imide, methods for the production of these compounds, medicaments containing these and their production as well as their therapeutic use, especially as cytostatic agents and immunosuppressive agents, for example in the treatment or prevention of various types of tumors, inhibition of abnormal cell growth and control of immune reactions, for example autoimmune diseases.
-
CYCLIC IMIDE-SUBSTITUTED PYRIDYLALKANE, ALKENE, AND ALKINE CARBOXAMIDES USEFUL AS CYTOSTATIC AND IMMUNOSUPPRESSIVE AGENTS申请人:Klinge Pharma GmbH公开号:EP1042315B1公开(公告)日:2004-04-14
-
Tertiary phosphine complexes of gold(I) and gold(III) with imido ligands: 1H, 31P, and 15N NMR spectroscopy, antiinflammatory activity, and x-ray crystal structure of (phthalimido)(triethylphosphine)gold(I)作者:Susan J. Berners Price、Michael J. DiMartino、David T. Hill、Reiko Kuroda、Muhammed A. Mazid、Peter J. SadlerDOI:10.1021/ic00215a026日期:1985.10
-
USE OF VITAMIN PP COMPOUNDS申请人:Klinge Pharma GmbH公开号:EP1079832B1公开(公告)日:2005-11-30
表征谱图
-
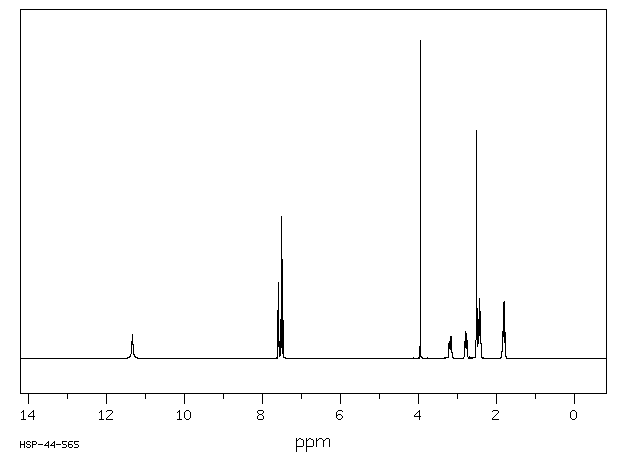
氢谱1HNMR
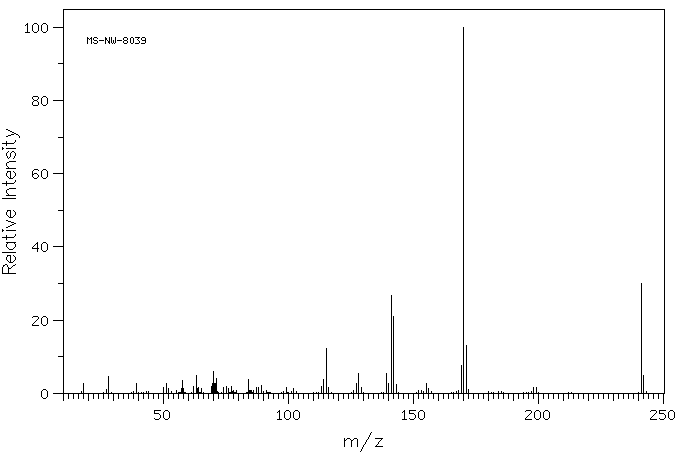
-
质谱MS
-
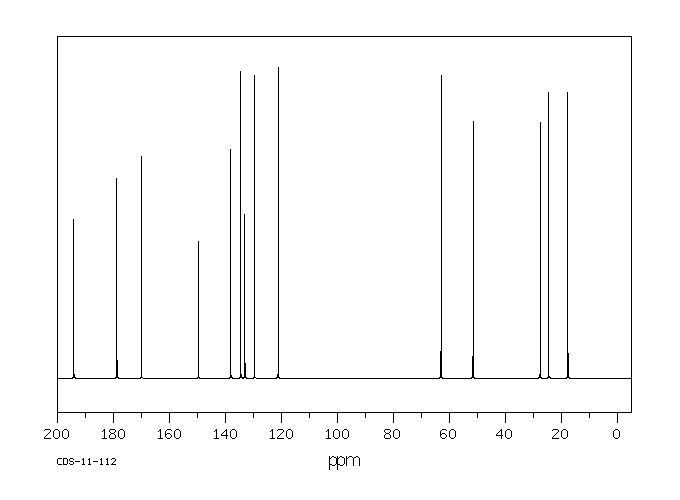
碳谱13CNMR
-
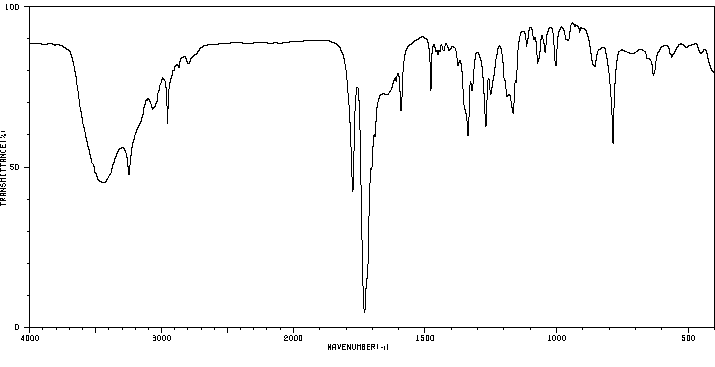
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-7,7-双[(4S)-(苯基)恶唑-2-基)]-2,2,3,3-四氢-1,1-螺双茚满
(R)-7,7-双[(4S)-(苯基)恶唑-2-基)]-2,2,3,3-四氢-1,1-螺双茚满
(4S,5R)-3,3a,8,8a-四氢茚并[1,2-d]-1,2,3-氧杂噻唑-2,2-二氧化物-3-羧酸叔丁酯
(3aS,8aR)-2-(吡啶-2-基)-8,8a-二氢-3aH-茚并[1,2-d]恶唑
(3aS,3''aS,8aR,8''aR)-2,2''-环戊二烯双[3a,8a-二氢-8H-茚并[1,2-d]恶唑]
(1α,1'R,4β)-4-甲氧基-5''-甲基-6'-[5-(1-丙炔基-1)-3-吡啶基]双螺[环己烷-1,2'-[2H]indene
齐洛那平
鼠完
麝香
风铃醇
颜料黄138
顺式-1,6-二甲基-3-(4-甲基苯基)茚满
雷美替胺杂质9
雷美替胺杂质24
雷美替胺杂质14
雷美替胺杂质13
雷美替胺杂质10
雷美替胺杂质
雷美替胺杂质
雷美替胺杂质
雷美替胺杂质
雷美替胺杂质
雷美替胺
雷沙吉兰相关化合物HCl
雷沙吉兰杂质8
雷沙吉兰杂质5
雷沙吉兰杂质4
雷沙吉兰杂质3
雷沙吉兰杂质16
雷沙吉兰杂质15
雷沙吉兰杂质12
雷沙吉兰杂质1
雷沙吉兰杂质
雷沙吉兰13C3盐酸盐
雷沙吉兰
阿替美唑盐酸盐
铵2-(1,3-二氧代-2,3-二氢-1H-茚-2-基)-8-甲基-6-喹啉磺酸酯
金粉蕨辛
金粉蕨亭
重氮正癸烷
酸性黄3[CI47005]
酒石酸雷沙吉兰
还原茚三酮(二水)
还原茚三酮
过氧化,2,3-二氢-1H-茚-1-基1,1-二甲基乙基
贝沙罗汀杂质8
表蕨素L
螺双茚满
螺[茚-2,4-哌啶]-1(3H)-酮盐酸盐
螺[茚-2,4'-哌啶]-1(3H)-酮