戊烷-3-硫醇 | 616-31-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-110.8°C
-
沸点:113.9°C
-
密度:0.837
-
LogP:2.66
-
物理描述:Clear to pale yellow liquid; Fruity roasted savoury aroma
-
溶解度:Very Slightly soluble in water
-
折光率:1.444 - 1.448
-
保留指数:758
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:6
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:1
-
氢给体数:1
-
氢受体数:1
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-(methylthio)pentane 57093-84-2 C6H14S 118.243
反应信息
-
作为反应物:参考文献:名称:Foldamers as Reactive Sieves: Reactivity as a Probe of Conformational Flexibility摘要:A series of m-phenyleneethynylene (mPE) oligomers modified with a dimethylaminopyridine (DMAP) unit were treated with methyl sulfonates of varying sizes and shapes, and the relative reactivities were measured by UV spectrophotometry. Using a small-molecule DMAP analogue as a reference, each of the methyl sulfonates was shown to react at nearly identical rate. In great contrast, oligomers that are long enough to fold, and hence capable of binding the methyl sulfonate, experience rate enhancements of 18-1600-fold relative to that of the small-molecule analogue, depending on the type of alkyl chain attached to the guest. Three different oligomer lengths were studied, with the longest oligomers exhibiting the fastest rate and greatest substrate specificity. Even large, bulky guests show slightly enhanced methylation rates compared to that with the reference DMAP, which suggests a dynamic nature to the oligomer's binding cavity. Several mechanistic models to describe this behavior are discussed.DOI:10.1021/ja067670a
-
作为产物:描述:参考文献:名称:[EN] CHEMICAL COMPOUNDS
[FR] COMPOSÉS CHIMIQUES摘要:本公开描述了新颖的化合物,或其药用可接受的盐,含有它们的药物组合物,以及它们的医疗用途。本公开的化合物具有作为Janus激酶(JAK)的双重调节剂的作用,单独使用,或与一个或多个附加机制(包括酪氨酸激酶,如TrkA或Syk,以及PDE4)结合使用,并且在治疗或控制炎症、自身免疫疾病、癌症以及其他调节JAK会可取的失调和其他适应症中是有用的。此外,还描述了通过施用本处所述的化合物来治疗炎症、自身免疫疾病、癌症以及其他易受JAK和PDE4抑制的状况的方法。公开号:WO2021003501A1
文献信息
-
Palladium-Catalyzed γ-C(sp<sup>3</sup> )−H Arylation of Thiols by a Detachable Protecting/Directing Group作者:Likun Jin、Jianchun Wang、Guangbin DongDOI:10.1002/anie.201807760日期:2018.9.17Reported herein is a palladium‐catalyzed, directed γ‐C(sp3)−H arylation of protected thiols. The key is to utilize Michael acceptors as a dual reagent to install a protecting/directing group on thiols by a thiol‐Michael click reaction, and remove it later under basic conditions. The C−H arylation proceeds with high functional‐group tolerance and the deprotected thiols can be further transformed into
-
The effect of substitutents at alkylsulfanyl/arylsulfanyl non-peripherally substituted phthalocyanines: Spectral and photophysical properties, basicity and photostability作者:Antonin Cidlina、Zuzana Pausimova、Miroslav Miletin、Petr Zimcik、Veronika NovakovaDOI:10.1142/s1088424615500832日期:2015.10
A series of magnesium, zinc and metal-free derivatives of non-peripherally substituted phthalocyanines (Pcs) bearing alkylsulfanyl or arylsulfanyl groups of different bulkiness was synthesized. Their spectral and photophysical properties including also the basicity of azomethine nitrogens and photostability were compared within the series as well as with similar peripherally substituted Pcs. Non-peripheral position of substituents led to the 70[Formula: see text]nm red-shift of Q-band in comparison to the peripherally substituted Pcs. However, unexpected blue-shift of approximately 50[Formula: see text]nm was observed in the series of non-peripherally substituted Pcs for the most bulky tert-butylsulfanyl derivative caused probably by extreme distortion of the macrocycle. The substitution had no effect on photophysical properties and compounds reached [Formula: see text] values 0.74–0.76 and [Formula: see text] 0.053–0.080 for zinc complexes, and [Formula: see text] 0.47–0.51 and [Formula: see text] 0.10–0.17 for magnesium complexes following the rule of heavy atom effect. Generally, non-peripherally substituted Pcs possessed improved singlet oxygen production in comparison to peripherally substituted ones. The photostability of the target compounds decreased with the red-shift of their absorption maxima with the arylsulfanyl derivatives being less photostable. The basicity of azomethine nitrogens was clearly dependent on the position and the character of substituent. Thus, non-peripherally substituted Pcs showed extraordinary increased basicity over the peripherally substituted ones with the most pronounced effect at alkylsulfanyl derivatives.
我们合成了一系列带有不同体积的烷基硫酰基或芳基硫酰基的非外周取代酞菁(Pcs)的镁、锌和无金属衍生物。对这些衍生物的光谱和光物理特性进行了比较,包括偶氮甲基硝基的碱性和光稳定性。与外围取代的 Pcs 相比,非外围位置的取代基导致 Q 波段发生了 70[式:见正文]纳米的红移。然而,在非外周取代的 Pcs 系列中,最粗大的叔丁基硫酰基衍生物出现了大约 50[式:见正文]纳米的意想不到的蓝移,这可能是由于大环的极度变形造成的。取代对光物理性质没有影响,锌络合物的[式:见正文]值为 0.74-0.76 和[式:见正文]0.053-0.080,镁络合物的[式:见正文]值为 0.47-0.51 和[式:见正文]0.10-0.17。一般来说,与外围取代的 Pcs 相比,非外围取代的 Pcs 能更好地产生单线态氧。目标化合物的光稳定性随着其吸收最大值的红移而降低,其中芳基硫代衍生物的光稳定性较差。偶氮甲基硝基的碱性明显取决于取代基的位置和性质。因此,非外周取代的 Pcs 比外周取代的 Pcs 具有更强的碱性,其中烷基硫衍生物的效果最为明显。 -
Reactions of 2-(α-Haloalkyl)thiiranes with nucleophilic reagents: V. Reactions of 2-(α-Chloroalkyl)thiiranes with organolithium compounds作者:A. A. Tomashevskii、V. V. Sokolov、A. A. PotekhinDOI:10.1134/s1070428010120080日期:2010.12corresponding allyl sulfides. The reactions of diastereoisomeric erythro- and threo-2-(1-chloroethyl)thiiranes with phenyllithium were stereospecific, and they afforded (E)- and (Z)-1-phenylsulfanylbut-2-enes, respectively. 3-Chloromethyl-2,2-dimethylthiirane and phenyllithium gave rise to a mixture of 3-methyl-3-phenylsulfanylbut-1-ene and 3-methyl-1-phenylsulfanylbut-2-ene. The reactions of 2-chloromethylthiiranes
-
Discovery and Lead Optimization of Benzene-1,4-disulfonamides as Oxidative Phosphorylation Inhibitors作者:Ding Xue、Yibin Xu、Armita Kyani、Joyeeta Roy、Lipeng Dai、Duxin Sun、Nouri NeamatiDOI:10.1021/acs.jmedchem.1c01509日期:2022.1.13aerobic metabolism. Here, we report the discovery, optimization, and structure–activity relationship (SAR) study of a series of novel OXPHOS inhibitors. The hit compound, benzene-1,4-disulfonamide 1, was discovered in a phenotypic screen selective for cytotoxicity in a galactose-containing medium. Our multi-parameter optimization campaign led to the discovery of 65 (DX3-235), showing nanomolar inhibition抑制氧化磷酸化(OXPHOS)对于依赖有氧代谢的特定癌症来说是一种很有前途的治疗策略。在这里,我们报告了一系列新型 OXPHOS 抑制剂的发现、优化和构效关系 (SAR) 研究。命中化合物苯-1,4-二磺酰胺1是在含半乳糖培养基中对细胞毒性有选择性的表型筛选中发现的。我们的多参数优化活动导致了65 ( DX3-235 ) 的发现,显示了在含半乳糖的培养基中对复合物 I 功能和三磷酸腺苷 (ATP) 产生的纳摩尔抑制,从而导致显着的细胞毒性。重要的是,64 ( DX3-234) 是65的紧密类似物,在小鼠中具有良好的耐受性,并且在 Pan02 同基因胰腺癌模型中显示出显着的单药疗效,这表明高效和选择性的 OXPHOS 抑制剂可用于治疗胰腺癌。
-
Multiparameter Optimization of Oxidative Phosphorylation Inhibitors for the Treatment of Pancreatic Cancer作者:Ding Xue、Yibin Xu、Armita Kyani、Joyeeta Roy、Lipeng Dai、Duxin Sun、Nouri NeamatiDOI:10.1021/acs.jmedchem.1c01934日期:2022.2.24Targeting oxidative phosphorylation (OXPHOS) complexes is an emerging strategy to disrupt the metabolism of select cancer subtypes and to overcome resistance to targeted therapies. Here, we describe our lead optimization campaign on a series of benzene-1,4-disulfonamides as novel OXPHOS complex I inhibitors. This effort led to the discovery of compound 23 (DX3-213B) as one of the most potent complex靶向氧化磷酸化 (OXPHOS) 复合物是一种新兴策略,可破坏选定癌症亚型的代谢并克服对靶向治疗的耐药性。在这里,我们描述了我们对一系列苯-1,4-二磺酰胺作为新型 OXPHOS 复合物 I 抑制剂的先导优化活动。这一努力导致发现化合物23 ( DX3-213B ) 作为迄今为止报道的最有效的复合物 I 抑制剂之一。DX3-213B破坏三磷酸腺苷 (ATP) 的生成,抑制复合物 I 功能,并导致胰腺癌细胞在低纳摩尔范围内的生长抑制。重要的是, DX3-213B的口服给药导致显着的体内在胰腺癌同基因模型中的疗效,没有明显的毒性。我们的数据清楚地表明,OXPHOS 抑制是治疗胰腺癌的一种安全有效的策略。
表征谱图
-
氢谱1HNMR
-
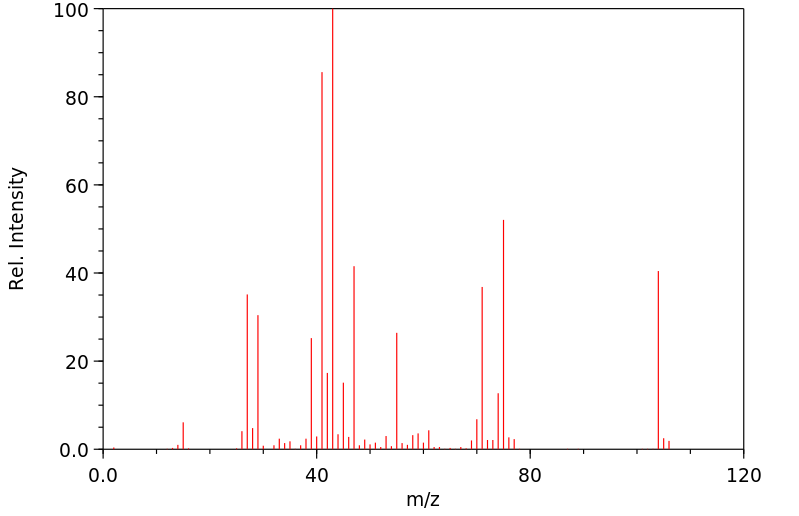
质谱MS
-
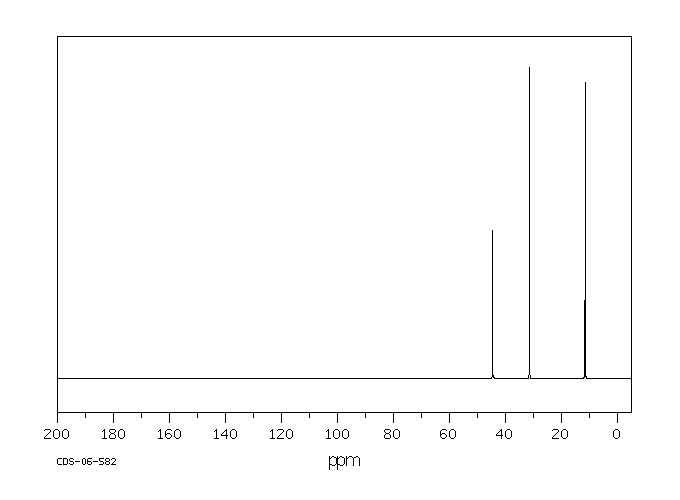
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息