2,3,5-三溴-4-甲基噻吩 | 67869-13-0
中文名称
2,3,5-三溴-4-甲基噻吩
中文别名
2,4,5-三溴-3-甲基噻吩
英文名称
2,3,5-tribromo-4-methylthiophene
英文别名
2,4,5-tribromo-3-methylthiophene
CAS
67869-13-0
化学式
C5H3Br3S
mdl
——
分子量
334.857
InChiKey
DTLNXOZXOZCNMX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:31°C
-
沸点:283.0±35.0 °C(Predicted)
-
密度:2.329±0.06 g/cm3(Predicted)
-
闪点:31 °C
-
稳定性/保质期:
远离氧化物、光和热。
计算性质
-
辛醇/水分配系数(LogP):4.7
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:28.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2934999090
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
SDS
2,3,5-Tribromo-4-methylthiophene Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,3,5-Tribromo-4-methylthiophene
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,3,5-Tribromo-4-methylthiophene
Percent: >95.0%(GC)
CAS Number: 67869-13-0
Chemical Formula: C5H3Br3S
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
2,3,5-Tribromo-4-methylthiophene
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a refrigerator.
Store away from incompatible materials such as oxidizing agents.
Heat-sensitive, Light-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: Crystal- Lump
Colour: White - Pale yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:31°C (Freezing point)
Boiling point/range: No data available
No data available
Flash point:
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: No data available
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
2,3,5-Tribromo-4-methylthiophene
Section 10. STABILITY AND REACTIVITY
Chemical stability: Labile.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Sulfur oxides, Hydrogen bromide
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
2,3,5-Tribromo-4-methylthiophene
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,3,5-Tribromo-4-methylthiophene
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,3,5-Tribromo-4-methylthiophene
Percent: >95.0%(GC)
CAS Number: 67869-13-0
Chemical Formula: C5H3Br3S
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
2,3,5-Tribromo-4-methylthiophene
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a refrigerator.
Store away from incompatible materials such as oxidizing agents.
Heat-sensitive, Light-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: Crystal- Lump
Colour: White - Pale yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:31°C (Freezing point)
Boiling point/range: No data available
No data available
Flash point:
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: No data available
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
2,3,5-Tribromo-4-methylthiophene
Section 10. STABILITY AND REACTIVITY
Chemical stability: Labile.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Sulfur oxides, Hydrogen bromide
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
2,3,5-Tribromo-4-methylthiophene
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,3-二溴-4-甲基噻吩 2,3-Dibrom-4-methylthiophen 125257-38-7 C5H4Br2S 255.961 2,4,-二溴-3-甲基噻吩 2,4,-dibromo-3-methylthiophene 53119-66-7 C5H4Br2S 255.961 2,4-二溴噻吩-3-甲腈 2,4-dibromothiophene-3-carbonitrile 1256651-68-9 C5HBr2NS 266.944 —— (2,4-dibromo-3-thienyl)methanol 1257070-65-7 C5H4Br2OS 271.96 —— 2,4-dibromo-3-(bromomethyl)thiophene 905838-63-3 C5H3Br3S 334.857 2,4-二甲基噻吩 2,4-dibromo-3,5-dimethylthiophene 63862-00-0 C6H6Br2S 269.988
反应信息
-
作为反应物:描述:参考文献:名称:Angeli; Ciamician, Chemische Berichte, 1891, vol. 24, p. 77摘要:DOI:
-
作为产物:参考文献:名称:使用季铵多卤化物的卤化。XXXI 噻吩衍生物与苄基三甲基多卤化铵的卤化摘要:噻吩衍生物与四氯碘酸苄基三甲基铵、三溴化苄基三甲基铵、二氯碘酸苄基三甲基铵在乙酸或乙酸中的反应...DOI:10.1246/bcsj.64.2566
文献信息
-
N-(HETERO)ARYL-PYRROLIDINE DERIVATIVES OF PYRAZOL-4-YL-PYRROLO[2,3-d]PYRIMIDINES AND PYRROL-3-YL-PYRROLO[2,3-d]PYRIMIDINES AS JANUS KINASE INHIBITORS申请人:Rodgers James D.公开号:US20100298334A1公开(公告)日:2010-11-25The present invention relates to N-(hetero)aryl-pyrrolidine derivatives of Formula I: which are JAK inhibitors, such as selective JAK1 inhibitors, useful in the treatment of JAK-associated diseases including, for example, inflammatory and autoimmune disorders, as well as cancer.
-
Substituted thienyl and furyl acylguanidines and methods of their use as beta-secretase modulators申请人:Fobare Floyd William公开号:US20060183792A1公开(公告)日:2006-08-17The present invention provides a substituted thienyl or furyl acylguanidine compound of formula I The present invention also provides methods for the inhibition of β-secretase (BACE) and for the treatment of β-amyloid deposits and neurofibrillary tangles.
-
Highly Regioselective Preparation of Heteroaryl-Magnesium Reagents by Using a Br/Mg Exchange作者:Christoph Sämann、Benjamin Haag、Paul KnochelDOI:10.1002/chem.201202230日期:2012.12.7Disubstituted thienyl‐, furyl‐ and pyridylmagnesium derivatives are regioselectively prepared from a Br/Mg exchange of the corresponding dibromo compounds by using either iPrMgCl⋅LiCl or hindered arylmagnesium reagents, such as isitylmagnesium bromide⋅lithium chloride (isityl=2,4,6‐triisopropyl‐phenyl) complexed with a diamine ligand, in difficult cases. The selective functionalisations of these heterocyclic
-
Synthesis and Relative Diatropicity of a Remarkably Aromatic Thia[13]annulene作者:Reginald H. Mitchell、Vivekanantan S. IyerDOI:10.1021/ja953025k日期:1996.1.14-Bis(bromomethyl)-3-methylthiophene was synthesised as an intermediate to prepare the strained thiophenobenzophanediene 11, which readily thermally valence isomerizes to the novel thia[13]annulene 10. This thia-annulene is notably diatropic and shows about 40% of the ring current of the parent bridged [14]annulene 9, which is substantially more than an analogous oxaannulene, 18, and substantially less than the azaannulene
-
Regioselective Bromine/Magnesium Exchange for the Selective Functionalization of Polyhalogenated Arenes and Heterocycles作者:Alexandre Desaintjean、Tobias Haupt、Leonie J. Bole、Neil R. Judge、Eva Hevia、Paul KnochelDOI:10.1002/anie.202012496日期:2021.1.18toluene enables efficient and regioselective Br/Mg exchanges with various dibromo‐arenes and ‐heteroarenes under mild reaction conditions and provides bromo‐substituted magnesium reagents. Assessing the role of Lewis donor additives in these reactions revealed that N,N,N′,N′′,N′′‐pentamethyldiethylenetriamine (PMDTA) finely tunes the regioselectivity of the Br/Mg exchange on dibromo‐pyridines and quinolines
表征谱图
-
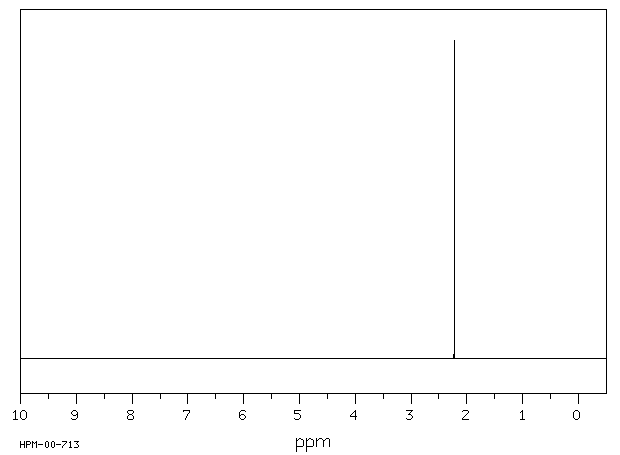
氢谱1HNMR
-
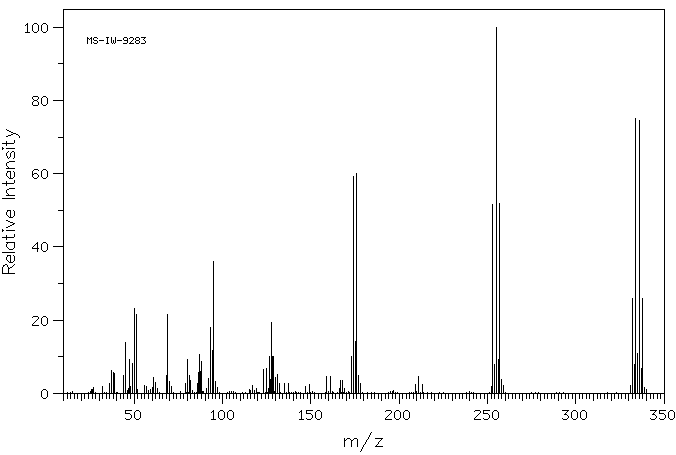
质谱MS
-
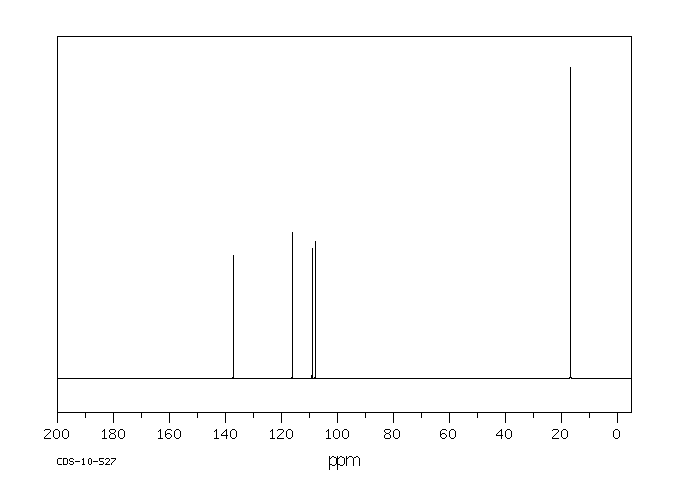
碳谱13CNMR
-
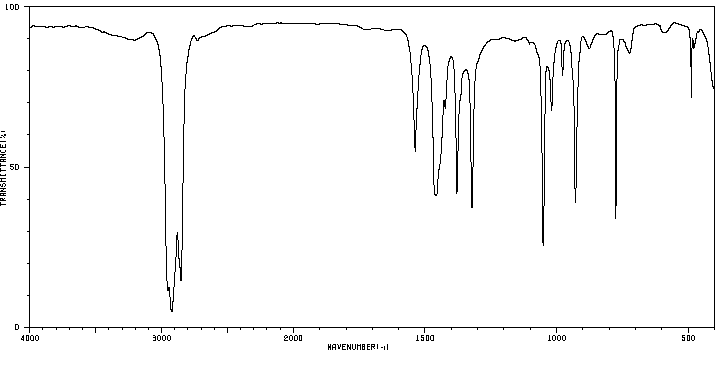
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
试剂2,5-Dibromo-3,4-dihexylthiophene
苯-1,2,4-三羧酸-丙烷-1,2,3-三醇(1:1)
碘吡咯
癸氯-二茂铁
甲酮,(4,5-二溴-1H-吡咯-2-基)苯基-
甲基3-氟-1H-1,2,4-三唑-5-羧酸酯
溴代二茂铁
溴-(3-溴-2-噻嗯基)镁
派瑞林D
派瑞林 F 二聚体
氯代二茂铁
曲洛酯
异噻唑,3-氯-5-甲基-
地茂酮
四碘硒吩
四碘噻吩
四碘呋喃
四溴噻吩
四溴吡咯
四溴-N-甲基吡咯
四氯噻吩
四氟噻吩
噻菌腈
噻美尼定.
噻吩,3-溴-4-(1-辛炔基)-
噻吩,3-溴-2-[2-(甲硫基)乙烯基]-,(Z)-
噻吩,3-溴-2-[2-(甲硫基)乙烯基]-,(E)-
噻吩,3-溴-2-[2-(甲硫基)乙烯基]-,(E)-
噻吩,2,5-二氯-3,4-二(氯甲基)-
喷贝特
咪唑烷,2-(4-溴-5-甲基-2-呋喃基)-1,3-二甲基-
叔丁基2-溴-4,6-二氢-5H-吡咯并[3,4-D]噻唑-5-羧酸酯
叔-丁基3-溴-6,7-二氢-1H-吡唑并[4,3-C]吡啶-5(4H)-甲酸基酯
叔-丁基2-溴-5,6-二氢咪唑并[1,2-A]吡嗪-7(8H)-甲酸基酯
叔-丁基(4-溴-5-氰基-1-甲基-1H-吡唑-3-基)氨基甲酯
双环[4.2.0]辛-1,3,5-三烯-7-甲腈,2-氟-
八氟联苯烯
八氟二苯并硒吩
全氟苯并环丁烯二酮
二苯基氯化碘盐
二联苯碘硫酸盐
二氯对二甲苯二聚体
二氯[2-甲基-3(2H)-异噻唑酮-O]的钙合物
二氯-1,2-二硫环戊烯酮
二-(3-溴-1,2,4-噻二唑-5-基)-二硫醚
二(2-噻吩基)碘鎓
乙酸,[[[1-(3-溴-5-异[口噁]唑基)亚乙基]氨基]氧代]-,甲基酯,(E)-
[四丁基铵][Δ-三(四氯-1,2-苯二醇酸根)磷酸盐(V)]
[3-(4-氯-3,5-二甲基-1H-吡唑-1-基)丙基]胺
[3-(4-氯-1H-吡唑-1-基)-2-甲基丙基]胺