ethyl 2-diazo-2-(phenylsulfonyl)acetate | 134919-31-6
中文名称
——
中文别名
——
英文名称
ethyl 2-diazo-2-(phenylsulfonyl)acetate
英文别名
ethyl phenylsulfonyl(diazo)acetate;ethyl 2-diazo-2-(benzenesulfonyl)acetate;ethyl (2Z)-2-(benzenesulfonyl)-2-diazoacetate
CAS
134919-31-6
化学式
C10H10N2O4S
mdl
——
分子量
254.266
InChiKey
RSQCRWJSEMIDQR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:17
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:70.8
-
氢给体数:0
-
氢受体数:5
上下游信息
反应信息
-
作为反应物:描述:ethyl 2-diazo-2-(phenylsulfonyl)acetate 在 dirhodium tetraacetate 、 sodium hydride 作用下, 以 N,N-二甲基甲酰胺 、 mineral oil 、 苯 为溶剂, 反应 2.5h, 生成 (±)-ethyl 2-(benzenesulfonyl)oxetane-2-carboxylate参考文献:名称:Synthesis of diversely functionalised 2,2-disubstituted oxetanes: fragment motifs in new chemical space摘要:新颖的氧杂环结构,其中环上包含多种功能基团,可以通过氧-氢插入/环化策略轻松获得。DOI:10.1039/c5cc05740j
-
作为产物:描述:苯砜乙酸乙酯 在 对甲苯磺酰叠氮 、 caesium carbonate 作用下, 以 四氢呋喃 为溶剂, 反应 0.5h, 以91%的产率得到ethyl 2-diazo-2-(phenylsulfonyl)acetate参考文献:名称:活性亚甲基化合物重氮转移反应的改进和有效方法摘要:摘要 在碳酸铯的存在下,活性亚甲基化合物和对甲苯磺酰叠氮化物之间的重氮转移反应可以有效地介导。DOI:10.1080/00397919508011762
文献信息
-
Benzotriazol-1-yl-sulfonyl Azide for Diazotransfer and Preparation of Azidoacylbenzotriazoles作者:Alan R. Katritzky、Mirna El Khatib、Oleg Bol’shakov、Levan Khelashvili、Peter J. SteelDOI:10.1021/jo101296s日期:2010.10.1Benzotriazol-1-yl-sulfonyl azide, a new crystalline, stable, and easily available diazotransfer reagent provides N-(α-azidoacyl)benzotriazoles convenient for N-, O-, C- and S-acylations. The efficient syntheses of various amides, azido protected peptides, esters, ketones and thioesters is reported together with a wide range of azides (including α-azido acids from α- amino acids in partially aqueous conditions) and
-
Copper-Catalyzed Regioselective Cascade Alkylation and Cyclocondensation of Quinoline <i>N</i> -Oxides with Diazo Esters: Direct Access to Conjugated π-Systems作者:Aniruddha Biswas、Ujjwal Karmakar、Arun Pal、Rajarshi SamantaDOI:10.1002/chem.201602493日期:2016.9.19An inexpensive copper‐catalyzed cascade regioselective alkylation, followed by cyclocondensation of quinoline N‐oxides with α‐diazo esters has been achieved successfully to provide heteroarene‐containing conjugated π‐systems. The developed method is simple, straightforward, and economical with a broad range of substrate scope. The dual role of copper catalyst in the C−H bond functionalization and in
-
Synthesis of Ionic Liquid-Supported Sulfonyl Azide and Its Application in Diazotransfer Reaction作者:Manoj Kumar Muthyala、Sunita Choudhary、Anil KumarDOI:10.1021/jo301529b日期:2012.10.5The paper describes synthesis of a novel ionic liquid-supported sulfonyl azide and its applications as diazotransfer reagent of active methylene compounds as well as deformylative diazo transfer reagent. The diazo compounds were isolated in excellent yields (82–94%) and high purity. The method offers better separation of product and reagent. This method is experimentally simple and mild, and requires
-
Design and synthesis of stable α-diazo-β-oxo sulfoxides作者:Stuart G. Collins、Orlagh C. M. O'Sullivan、Patrick G. Kelleher、Anita R. MaguireDOI:10.1039/c3ob27061k日期:——Diazo transfer adjacent to a sulfoxide moiety to provide stable, isolable α-diazo-β-oxo sulfoxides has been achieved. Use of monocyclic and bicyclic sulfoxide precursors is critical in enabling isolation of stable derivatives, through introduction of conformational constraint, while acyclic α-diazo-β-oxo sulfoxides are too labile to isolate and characterize.已经实现了与亚砜部分相邻的重氮转移,以提供稳定的,可分离的α-重氮-β-氧代亚砜。使用单环和双环亚砜前体对于通过引入构象约束条件分离稳定的衍生物至关重要,而无环α-重氮-β-氧代亚砜过于不稳定,无法分离和表征。
-
C6-Selective Direct Alkylation of Pyridones with Diazo Compounds under Rh(III)-Catalyzed Mild Conditions作者:Debapratim Das、Aniruddha Biswas、Ujjwal Karmakar、Santanu Chand、Rajarshi SamantaDOI:10.1021/acs.joc.5b02349日期:2016.2.5A Rh(III)-catalyzed highly efficient C6-alkylation of 2-pyridones has been achieved successfully with α-diazo carbonyl compounds. The developed method is simple, mild, and highly regioselective with a broad range of substrate scope. The regioselectivity is guided by the pyridyl substituent attached to the nitrogen center of the pyridone ring. The directing group can be easily removed, and the only
表征谱图
-
氢谱1HNMR
-
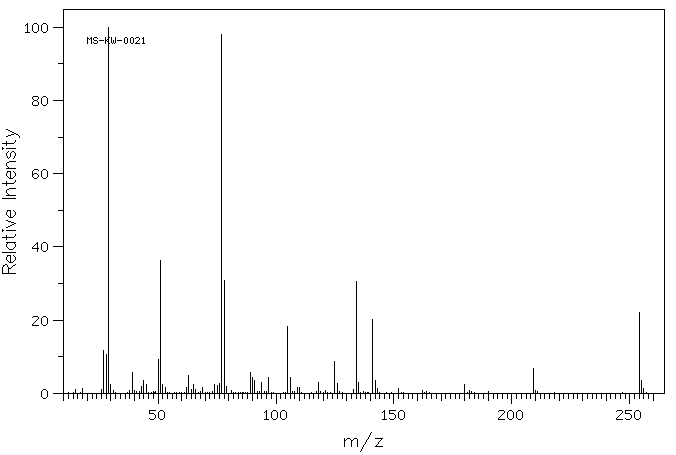
质谱MS
-
碳谱13CNMR
-
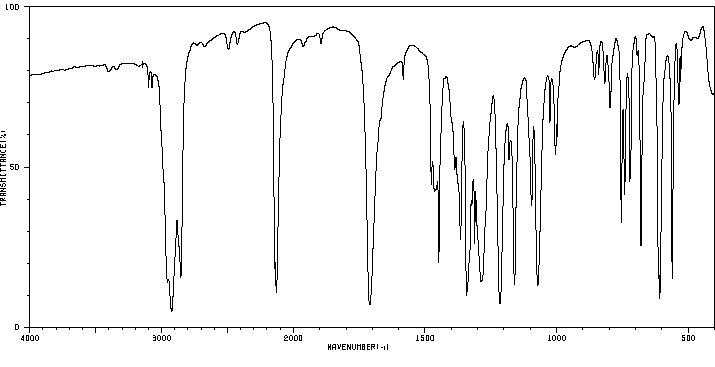
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫