3-甲基-2-丁硫醇 | 2084-18-6
中文名称
3-甲基-2-丁硫醇
中文别名
3-甲基-2-丁烷硫醇;仲异戊硫醇
英文名称
3-methylbutane-2-thiol
英文别名
3-methyl-2-butanethiol;3-Methyl-2-butanthiol
CAS
2084-18-6
化学式
C5H12S
mdl
MFCD00039648
分子量
104.216
InChiKey
BFLXFRNPNMTTAA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-109.95°C (estimate)
-
沸点:109-112 °C(lit.)
-
密度:0.841 g/mL at 25 °C(lit.)
-
闪点:60 °F
-
LogP:2.47
-
物理描述:colourless liquid with repulsive, mercaptan-like odour
-
溶解度:insoluble in water, miscible with alcohol and ether
-
折光率:1.442-1.452
-
保留指数:743
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:1
-
氢给体数:1
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:F
-
安全说明:S16,S33,S36
-
危险类别码:R11
-
WGK Germany:3
-
海关编码:2930909090
-
危险品运输编号:UN 3336 3/PG 2
-
包装等级:II
-
危险类别:3
-
危险性防范说明:P210
-
危险性描述:H225
SDS
3-甲基-2-丁硫醇 修改号码:5
模块 1. 化学品
产品名称: 3-Methyl-2-butanethiol
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第2级
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 高度易燃液体和蒸气
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
3-甲基-2-丁硫醇 修改号码:5
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3-甲基-2-丁硫醇
百分比: >98.0%(GC)
CAS编码: 2084-18-6
俗名: 3-Methyl-2-butyl Mercaptan
分子式: C5H12S
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
3-甲基-2-丁硫醇 修改号码:5
模块 8. 接触控制和个体防护
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-极淡的黄色
气味: 恶臭味
pH: 无数据资料
熔点: 无资料
沸点/沸程 110 °C
闪点: 16°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.84
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
3-甲基-2-丁硫醇 修改号码:5
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧,焚烧时需要特别注
意该物质是高度可燃的。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 3336
正式运输名称: 硫醇, 液体, 易燃的, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 3-Methyl-2-butanethiol
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第2级
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 高度易燃液体和蒸气
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
3-甲基-2-丁硫醇 修改号码:5
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3-甲基-2-丁硫醇
百分比: >98.0%(GC)
CAS编码: 2084-18-6
俗名: 3-Methyl-2-butyl Mercaptan
分子式: C5H12S
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
3-甲基-2-丁硫醇 修改号码:5
模块 8. 接触控制和个体防护
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-极淡的黄色
气味: 恶臭味
pH: 无数据资料
熔点: 无资料
沸点/沸程 110 °C
闪点: 16°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.84
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
3-甲基-2-丁硫醇 修改号码:5
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧,焚烧时需要特别注
意该物质是高度可燃的。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 3336
正式运输名称: 硫醇, 液体, 易燃的, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
反应信息
-
作为反应物:描述:参考文献:名称:THIO(DI)SILANES摘要:含有化学式(A)的硫代硅烷类化合物:(R1aR1bR1cCS)s(Si)XxHh(A),其中下标s为2至4,或者含有化学式(I)的硫代硅烷类化合物:(R1aR1bR1cCS)s(R22N)(Si—Si)XxHh(I),其中下标s为1至6,其中R1a、R1b、R1c、R2、X和下标n、x和h在此定义。还包括含有相同成分的组合物,制备和使用方法,合成中有用的中间体,以及由此制备的薄膜和材料。公开号:US20200024291A1
-
作为产物:描述:参考文献:名称:硫化NiMo / Al 2 O 3对仲和叔α-碳原子烷基胺加氢脱氮的机理。摘要:具有仲和叔α-碳原子的烷基胺的加氢脱氮(HDN)(2-戊胺,3-甲基-2-丁胺,3,3-二甲基-2-丁胺,2-甲基环己胺,2-甲基-2-丁胺)在硫化的NiMo / Al 2 O 3上研究了苄胺和相应的烷硫醇的加氢脱硫(HDS)。烷硫醇和二烷基胺是具有二级α-碳原子的胺的HDN中的主要产物,是通过H 2取代胺基而形成的S或烷基胺。烷烃和烯烃是由链烷硫醇的消除和氢解反应形成的副产物,这已通过烷基胺的HDN和相应链烷硫醇的HDS中相似的烯烃/烷烃比得到证实。2-甲基-2-丁胺和苄胺的反应比具有仲α-碳原子的胺反应快得多。甲基丁烯和甲基丁烷是2-甲基-2-丁胺的主要产物,甲苯是苄胺的主要产物。该和2-甲基-2-丁胺的HDN和2-甲基-2-丁硫醇的HDS中不同的甲基丁烯/甲基丁烷比率表明,具有叔α-碳原子的2-甲基-2-丁胺已被活化。苄胺通过E1机理反应。DOI:10.1016/j.jcat.2003.12.013
文献信息
-
C-S cross-coupling of aryl halides with alkyl thiols catalyzed by in-situ generated nickel(II) N-heterocyclic carbene complexes作者:Fang-Jie Guo、Jing Sun、Zhao-Qing Xu、Fritz E. Kühn、Shu-Liang Zang、Ming-Dong ZhouDOI:10.1016/j.catcom.2017.02.007日期:2017.6C-S cross-coupling of aryl halides with alkyl thiols catalyzed by in-situ generated Ni (II) N-heterocyclic carbene (NHC) complexes is investigated. Good to excellent yields can be obtained for a variety of aryl halides when using 5 mol% of the Ni (II)-NHC catalyst and 1.5 eq. of KOtBu. Both the electronic and steric effects of the NHC ligands on the catalytic performance of Ni (II)-NHC, as well as the
-
Highly Efficient and Chemoselective Tertiary and Secondary Benzylation of Thiols Catalyzed by Indium(III) Triflate作者:Krzysztof Kuciński、Grzegorz HreczychoDOI:10.1002/ejoc.201701007日期:2017.10.10We have developed a highly efficient method for the chemoselective nucleophilic substitution of tertiary and secondary benzylic alcohols with aliphatic and aromatic thiols in the presence of catalytic amounts of indium(III) triflate under mild conditions. A broad range of unsymmetrical sulfides were synthesized in excellent isolated yields (89–99 %) by using this approach.
-
P(OEt)3-Mediated Formal S–H Insertion: Reductive Couplings of Isatins with Thiols to Generate 3-Sulfenylated Oxindoles作者:Dulin Kong、Mingshu Wu、Tiao Huang、Li Liu、Qinghe WangDOI:10.1055/s-0040-1707147日期:2020.9Abstract A new P(OEt)3-mediated formal S–H bond-insertion reaction of isatins into thiols for the synthesis of valuable 3-sulfenylation oxindoles has been developed. This approach takes advantage of the unique reactivity of Kukhtin–Ramirez adducts to allow direct reductive S–H functionalization with commercially available and bench-stable starting materials.
-
C-3取代的-9-脱氧-9A-氮杂-9A-高红霉素A衍 生物
-
Palladium-Catalyzed γ-C(sp<sup>3</sup> )−H Arylation of Thiols by a Detachable Protecting/Directing Group作者:Likun Jin、Jianchun Wang、Guangbin DongDOI:10.1002/anie.201807760日期:2018.9.17Reported herein is a palladium‐catalyzed, directed γ‐C(sp3)−H arylation of protected thiols. The key is to utilize Michael acceptors as a dual reagent to install a protecting/directing group on thiols by a thiol‐Michael click reaction, and remove it later under basic conditions. The C−H arylation proceeds with high functional‐group tolerance and the deprotected thiols can be further transformed into
表征谱图
-
氢谱1HNMR
-
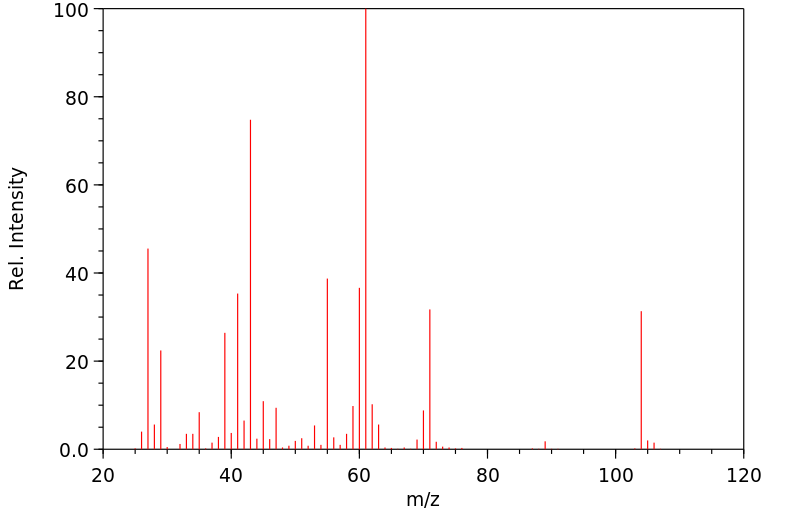
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
铜,丙烷-2-硫醇
铅,丙烷-1-硫醇
苏-(2R,4R)-戊二硫醇
羟基-乙醛
硫甘油
癸烷-2-硫醇
甲硫醇铅
甲硫醇钠
甲硫醇-d4
甲硫醇-S-d
甲硫醇
甲三硫醇
环辛硫醇
环戊硫醇
环戊基甲硫醇
环庚烷-1,1-二硫醇
环己硫醇
环己烷-1,1-二硫醇
环己基甲硫醇
环十二烷硫醇
环丙硫醇
环丙基甲硫醇
环丁硫醇
油烯基硫醇
氟甲硫醇
氘代甲硫醇-D3
正十四烷基硫醇
末端脱氧核苷酸转移酶
戊赤藓四硫醇
戊烷-3-硫醇
异戊硫醇
异戊烯基硫醇
异丙硫醇
异丁硫醇
庚-3-烯-4-硫醇
己-2-烯-1-硫醇
巯基甲烷-13C
巯基乙醛
巯基乙胺氢溴酸盐
巯基乙胺
巯基-十一胺盐酸盐
壬烷-2-硫醇
吡啶,2-(戊基硫代)-,1-氧化
叔壬基硫醇
叔十六硫醇
叔十二烷硫醇
叔丁基硫醇
反式-2-丁烯-1-硫醇
双(巯基环戊烷)四氯化钛
半胱胺盐酸盐