(+/-)-3-cyano-2-phenylpropionic acid | 442542-97-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:97 °C(Solv: benzene (71-43-2))
-
沸点:365.4±30.0 °C(Predicted)
-
密度:1.216±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:61.1
-
氢给体数:1
-
氢受体数:3
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 3-cyano-2-phenylpropanoate 88970-65-4 C11H11NO2 189.214 —— 3-Cyano-2-phenylpropanoyl chloride 674776-31-9 C10H8ClNO 193.633 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (R)-3-cyano-2-phenylpropionic acid 674776-40-0 C10H9NO2 175.187 —— (S)-3-Cyano-2-phenylpropanoic acid 177031-01-5 C10H9NO2 175.187 3-氰基-2-苯基丙酸乙酯 3-Aethoxycarbonyl-3-phenyl-propionitril 6840-18-2 C12H13NO2 203.241 4-氨基-2-苯基丁酸 (+/-)-4-amino-2-phenylbutanoic acid 13080-10-9 C10H13NO2 179.219 —— (R)-4-amino-2-phenylbutanoic acid 674776-49-9 C10H13NO2 179.219 —— (S)-4-amino-2-phenylbutanoic acid 177187-82-5 C10H13NO2 179.219 —— 3-Cyano-2-phenylpropanoyl chloride 674776-31-9 C10H8ClNO 193.633 4-[(叔丁氧羰基)氨基]-2-苯基丁酸 4-((tert-butoxycarbonyl)amino)-2-phenylbutanoic acid 790227-48-4 C15H21NO4 279.336
反应信息
-
作为反应物:描述:(+/-)-3-cyano-2-phenylpropionic acid 在 palladium on activated charcoal 氢气 、 溶剂黄146 作用下, 20.0 ℃ 、275.8 kPa 条件下, 反应 72.0h, 以99%的产率得到4-氨基-2-苯基丁酸参考文献:名称:γ-氨基酸的有效合成并尝试驱动其对映选择性。摘要:将羧酸双阴离子与溴乙腈铅加成,以良好的收率生成相应的γ-氰酸,其在氢化后产生γ-氨基酸。此两步方法改进了先前描述的结果。ee 的差是由于我们尝试通过手性酰胺诱导来驱动这种转化的对映选择性。DOI:10.3390/molecules13040716
-
作为产物:描述:(3R)-4,4-dimethyl-2-oxo-1-phenylpyrrolidin-3-yl 3-cyano-2-phenylpropionate 在 lithium hydroxide 作用下, 以 四氢呋喃 、 水 为溶剂, 生成 (+/-)-3-cyano-2-phenylpropionic acid参考文献:名称:Stereoselective synthesis of both enantiomers of N-Boc-α-aryl-γ-aminobutyric acids摘要:Esterification of racemic alpha-aryl-beta-cyanopropionic acid chlorides with either (R)- or (S)-N-phenylpantolactam as the chiral auxiliary in the presence of Et3N resulted in the predominant formation of (alphaR,3'R)- or (alphaS,3'S)-configured pantolactam cyano ester, respectively, in nearly quantitative yields with diastereomeric ratios of up to 93:7. Column chromatography of the diastereoenriched cyano esters, followed by hydrolysis of the resulting diastereopure cyano esters under essentially nonracemizing conditions gave enantiopure alpha-aryl-beta-cyanopropionic acids, which were readily converted in high yields into enantiopure (OER)- and (alphaS)-configured N-tert-butoxycarbonyl-alpha-aryl-gamma-aminobutyric acid (GABA) derivatives of potential biological interest. (C) 2003 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetasy.2003.10.035
文献信息
-
Practical Synthesis of Diethyl Phenylsuccinate by Mg-promoted Carboxylation of Ethyl Cinnamate作者:Hirofumi Maekawa、Taro Murakami、Takeshi Miyazaki、Ikuzo NishiguchiDOI:10.1246/cl.2011.368日期:2011.4.5Mg-promoted reduction of ethyl cinnamate (1a) in the presence of carbon dioxide gave a mixture of β-carboxylated compound 2a and α,β-dicarboxylated compound 3a. Similar reductive carboxylation of 1...
-
Electrochemical Reduction of Cinnamonitrile in the Presence of Carbon Dioxide: Synthesis of Cyano- and Phenyl-Substituted Propionic Acids作者:Huan Wang、Mei-Yu Lin、Kai Zhang、Su-Jiao Li、Jia-Xing LuDOI:10.1071/ch08092日期:——
Cyano- and phenyl-substituted propionic acids were synthesized simply and efficiently by electrocarboxylation of cinnamonitrile in undivided cells using the non-noble metal nickel as cathode and magnesium as the anode. The radical anion generated by the electroreduction of cinnamonitrile in the absence of CO2 is involved in several competitive reactions that lead to the formation of linear hydrodimers, cyclic hydrodimers, saturated dihydro products, and glutaronitrile derivatives. While under 101.325 kPa of CO2, the electrocarboxylation could easily be carried out in the absence of additional catalysts, and with good yield (84.8%). The influence of various synthetic parameters, such as the nature of the electrode, the working potential, the concentration, and the temperature, on the electrocarboxylation reaction was investigated.
-
ANTIVIRAL AGENTS申请人:Kumar V. Dange公开号:US20070287699A1公开(公告)日:2007-12-13Compounds are provided having utility for the treatment of viral infections, particularly HCV.提供了化合物,可用于治疗病毒感染,尤其是HCV。
-
Tetrahydroquinoline derivatives useful for neurodegenerative disorders申请人:MERCK SHARP & DOHME LTD.公开号:EP0386839A2公开(公告)日:1990-09-12A class of 1,2,3,4-tetrahydroquinolines possessing at least one substituent, or a spirocyclic moiety, at the 4-position, and an acidic group or a group convertible thereto in vivo at the 2-position, are specific antagonists of N-methyl-D-aspartate (NMDA) receptors and are therefore useful in the treatment and/or prevention of neurodegenerative disorders..一类1,2,3,4-四氢喹啉类化合物在4-位上具有至少一个取代基或螺环分子,在2-位上具有酸性基团或在体内可转化的基团,是N-甲基-D-天冬氨酸(NMDA)受体的特异性拮抗剂,因此可用于治疗和/或预防神经退行性疾病。
-
A simple synthesis of γ-aminoacids作者:Salvador Gil、Margarita Parra、Pablo RodríguezDOI:10.1016/j.tetlet.2007.03.037日期:2007.5The addition of dianions of carboxylic acids to bromoacetonitrile, leads, in good yields, to the corresponding gamma-cyanoacids that give gamma-aminoacids on hydrogenation. This two-step methodology improves the results previously described. (c) 2007 Elsevier Ltd. All rights reserved.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
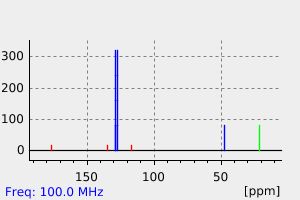
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息