2,4-dimethyl-1-penten-3-ol | 103668-38-8
中文名称
——
中文别名
——
英文名称
2,4-dimethyl-1-penten-3-ol
英文别名
2,4-dimethylpent-1-en-3-ol
CAS
103668-38-8
化学式
C7H14O
mdl
——
分子量
114.188
InChiKey
DWLSOADTNMPXFH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.71
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
反应信息
-
作为反应物:描述:参考文献:名称:Umnowa, Zhurnal Russkago Fiziko-Khimicheskago Obshchestva, 1910, vol. 42, p. 1533摘要:DOI:
-
作为产物:描述:参考文献:名称:氢过氧化物的烯丙基重排:烯丙基过氧自由基摘要:己烯丙基过氧化氢(1)或异戊烯基氢过氧化物(2)在己烷中的自由基重排产生两种氢过氧化物的平衡混合物。碳骨架重排的缺乏表明参与氢过氧化物异构化的中间自由基的不成对电子位于除α-碳原子之外的其他位置。在烯丙基重排过程中,氧不能与松香,烯丙基和无环氢过氧化物(8)和(9)反应,这支持了这一建议。1-异丙基-2-甲基烯丙基氢过氧化物(8)的重排动力学与其中中间体是直接由任一烯丙基异构体产生的常见烯丙基过氧自由基的机理相容。DOI:10.1039/p29840000621
文献信息
-
The Exceptional Chelating Ability of Dimethylaluminum Chloride and Methylaluminum Dichloride. The Merged Stereochemical Impact of α- and β-Stereocenters in Chelate-Controlled Carbonyl Addition Reactions with Enolsilane and Hydride Nucleophiles作者:David A. Evans、Brett D. Allison、Michael G. Yang、Craig E. MasseDOI:10.1021/ja011337j日期:2001.11.1beta-disubstituted aldehydes concludes that the syn aldehyde diastereomer possesses the arrangement of stereocenters wherein the alpha- and beta-substituents impart a reinforcing facial bias upon the aldehyde carbonyl. Aldol reactions of syn aldehydes were thus observed to proceed with uniformly excellent diastereofacial selectivity. Aldol reactions of the corresponding anti aldehydes containing opposing
-
The kinetic resolution of allylic alcohols by a non-enzymatic acylation catalyst; application to natural product synthesis作者:Stéphane Bellemin-Laponnaz、Jennifer Tweddell、J. Craig Ruble、Frank M. Breitling、Gregory C. FuDOI:10.1039/b002041i日期:——A planar-chiral DMAP derivative is shown to serve as an effective catalyst for the kinetic resolution of allylic alcohols; to illustrate its practical utility, the catalyst is applied to the resolution of two alcohols that have been employed as intermediates in recent natural product total syntheses.
-
Unusual E-Selective Ring-Closing Metathesis To Form Eight-Membered Rings作者:Ryosuke Matsui、Kentaro Seto、Kazuhiro Fujita、Takahiro Suzuki、Atsuo Nakazaki、Susumu KobayashiDOI:10.1002/anie.201004746日期:2010.12.27Tied back: The title reaction was observed when a silicon‐tethered diene was treated with the Hoveyda–Grubbs second‐generation catalyst. The structural requirements for the E‐olefin‐forming ring‐closing metathesis, and the transition state leading to E olefin are discussed. This methodology will be useful in the synthesis of polyketides containing a pent‐2‐ene‐1,5‐diol unit.
-
Stereoselective Synthesis of cis-β-Methyl- and Phenyl-Substituted Alkenylboronates by Platinum-Catalyzed Dehydrogenative Borylation作者:Toshimichi Ohmura、Yuta Takasaki、Hideki Furukawa、Michinori SuginomeDOI:10.1002/anie.200805406日期:2009.3.16Changing places: Intramolecular B(pin)/H exchange took place in the presence of a platinum–phosphane catalyst, giving synthetically useful cis‐β‐methyl‐substituted alkenylboronates stereoselectively (see scheme; B(pin)=4,4,5,5‐tetramethyl‐1,3,2‐dioxaborolan‐2‐yl).
-
Stereoselective synthesis of 4-alkoxy-3-methylidenealkanols using reactions between 2-(1-alkoxyalkyl)propenylstannanes and aldehydes: X-ray crystal structure of (1R,4R)-3-methylidene-1-(4-nitrophenyl)pentane-1,4-diol作者:Pedro Almendros、Michelangelo Gruttadauria、Madeleine Helliwell、Eric J. ThomasDOI:10.1039/a702256e日期:——The 2-(1-hydroxy- and 1-alkoxy-alkyl)propenylstannanes 9 and 11–15, react with aldehydes to form 4-hydroxy- and 4-alkoxy-3-methylidenealkanols 23, 24 and 36–53. The stereoselectivity of these reactions has been investigated. If the reactions are carried out by transmetallation of the stannane using a tin(IV) halide before addition of the aldehyde, modest stereoselectivity in favour of the 1,4-anti-products 23, 36 and 37 is observed for the hydroxystannane 9, whereas the alkoxystannanes 11–15 give rise preferentially to the 1,4-syn-diastereoisomers 47–53, selectivity 75–85∶25–15. It should be noted that these stereochemical assignments are the reverse of those reported in the preliminary communication on this work. The structure of the 1,4-anti-product 36 from the reaction between the hydroxystannane 9 and p-nitrobenzaldehyde was established by X-ray diffraction. The stereoselectivity of BINOL–titanium(IV) catalysed reactions of the (R)-SEM-stannane (R)-12 with benzaldehyde is controlled by the configuration of the BINOL and can be used to synthesize either the 1,4-anti- or 1,4-syn-isomers 40 and 47.2-(1-羟基-和1-烷氧基-烷基)丙烯基锡化合物9和11–15与醛反应生成4-羟基-和4-烷氧基-3-亚甲基烷基醇23、24和36–53。这些反应的立体选择性已被研究。如果反应通过锡化合物的转移金属化作用,使用四价锡卤化物,然后再加入醛进行反应,对于羟基锡化合物9,观察到适度的立体选择性,偏向于1,4-反式产物23、36和37,而烷氧基锡化合物11–15则优先生成1,4-顺式非对映异构体47–53,选择性为75–85∶25–15。值得注意的是,这些立体化学的指定与初步通讯中报道的情况相反。通过X射线衍射确定了羟基锡化合物9与对硝基苯甲醛反应生成的1,4-反式产物36的结构。BINOL-钛(IV)催化的(R)-SEM-锡化合物(R)-12与苯甲醛反应的立体选择性受BINOL构型的控制,可用于合成1,4-反式或1,4-顺式异构体40和47。
表征谱图
-
氢谱1HNMR
-
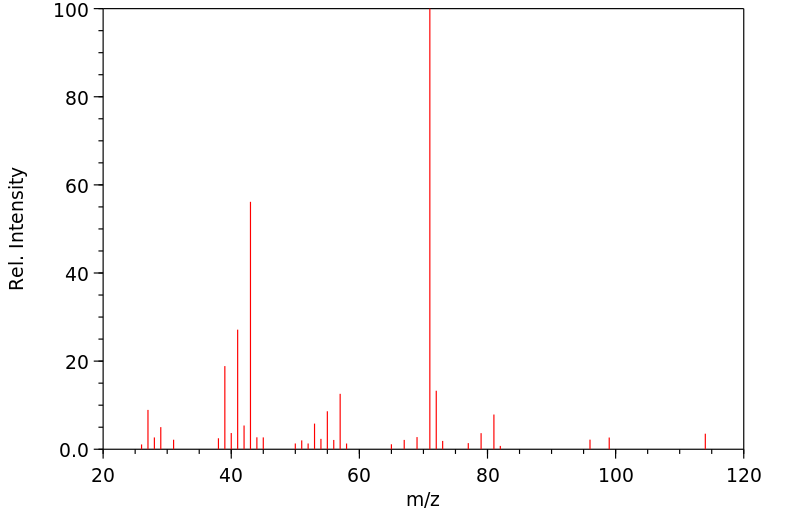
质谱MS
-
碳谱13CNMR
-
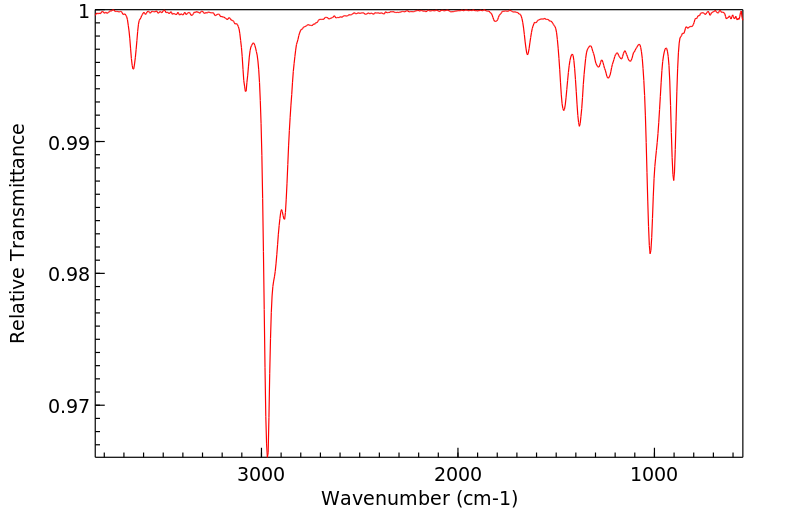
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷